Translate this page into:
Prognostic significance of age in traumatic brain injury
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Age is a strong prognostic factor following traumatic brain injury (TBI), with discrepancies defining the critical prognostic age threshold. This study was undertaken to determine the impact of various age thresholds on outcome after TBI.
Materials and Methods:
The ages of patients admitted with TBI were prospectively studied in relation to mode of injury, Glasgow coma score (GCS), CT category and surgical intervention. Mortality was assessed at 1 month, and neurological outcome was assessed at 6 months. Appropriate statistical analyzes (details in article) were performed.
Results:
Of the total 244 patients enrolled, 144 patients had severe, 38 patients had moderate and 62 patients had mild TBI, respectively. Age had significant association with grade of injury, CT category and surgical intervention (P < 0.01). Mortality at 1 month was significantly associated with increasing age with patients dead at 1 month being 15% for age < 18, 44% for age between 18 and 59 years, and 52% in the age group > 59 years respectively (P < 0.001). Unfavorable outcome showed significant association with an increase in age, every decade (P < 0.001). In multivariate analysis, there was stepwise increase in the odds of unfavorable outcome across age groups centered on 40 years, independent of confounding factors. The adjusted odds ratios for unfavorable outcome with regard to age thresholds 30, 40 and 50 years were 11.3, 53.3 and 1171, respectively (P < 0.005). Moreover, there was significant association of unfavorable outcome with age > 40 years in all subgroups, based on GCS and surgical intervention (P < 0.05).
Conclusions:
In patients with TBI, age demonstrates independent association with unfavorable outcome at 6 months, in stepwise manner centered on a threshold of 40 years.
Keywords
Age
brain injury
mortality
outcome
threshold
Introduction
Traumatic brain injury (TBI) is a major cause of disability, death and economic cost to our society.[12] Age has been found to be a strong prognostic factor, following TBI in various studies.[3–6] Compared to other well-accepted prognostic variables with definite cut-points (such as, GCS and CT category), there are striking discrepancies in the literature in defining the age point where prognosis significantly worsens, and the strength of the association is yet to be investigated across different age thresholds.[7] This study was undertaken to evaluate the influence of different age thresholds on various clinical and radiological parameters, mortality and neurological outcome at 6 months following TBI.
Materials and Methods
Patients admitted with head injury under the neurosurgical department of All India Institute of Medical Sciences, New Delhi, from June 2005 to December 2005 were taken up for the study. They were grouped into mild, moderate and severe injury, based on post-resuscitation admission Glasgow Coma Score (GCS)[8] of 13 - 15, 9 - 12 and 3 - 8, respectively[1]. All of them underwent computed tomography (CT) scan on admission and were classified into 1 of the 5 categories, based on increasing mass effect as per Traumatic Coma Data Bank (TCDB) study by Marshall et al.[9]
Standard care consisted of ventilation in patients with severe TBI, seizure prophylaxis with Phenytoin, gastric ulcer prophylaxis with Ranitidine. Mannitol was given to patients with CT, having evidence of focal mass effect or diffuse edema. Frusemide was added to patients with midline shift (> 5 mm). Fluid and electrolyte homeostasis was maintained. Decision regarding ICP monitoring and surgical decompression was taken according to the mass effect noted in CT and was individualized to each patient. Age, mode of injury, GCS, TCDB CT category, surgical decompression and other clinical parameters were noted in a pre-planned prospective database and were followed up.
The primary outcome was Glasgow Outcome Scale (GOS)[10], assessed at 3 and 6 months following injury, either directly or over telephone. Good recovery or moderate disability was considered as favorable outcome, and severe disability, persistent vegetative state or death was considered as unfavorable outcome. The secondary outcome assessed was mortality at 1 month.
SPSS software (version 10, SPSS Inc, Chicago) was used for the statistical analyses. Continuous variables in more than 2 groups were compared by using one-way analysis of variance (ANOVA). Proportions were compared by using chi-square tests or Fisher's exact test, depending on expected number in crosstab. As age is both a continuous scale variable and categorical ordinal variable based on various cut-offs, both kinds of analyses were performed. Multivariate analysis was also conducted using binary logistic regression with respect to age as a continuous variable as well as various age threshold ordinates, adjusting for admission GCS, TCDB CT category and surgical intervention. Two sided significance tests were used throughout, and the significance level was kept at P < 0.05.
Results
A total of 244 patients of TBI were included for the study. Their ages ranged from 1 to 80 years (median 30 years). There were 214 males and 30 females, with the ratio of 7:1. The predominant mode of injury was motor vehicle accidents involving 67% of patients, followed by falls from height (28%), and the rest 5% included assault, fall of objects, etc. The distribution of various modes of injury in different age groups is as shown [Figure 1]. Falls were more frequent in children and elderly, compared with others (P < 0.001). This may be due to ignorance of risks in children, and incoordination in elderly.

- Mode of injury in different age groups (P < 0.001)
Among the total 244 patients, 25% patients had mild, 16% patients had moderate and 59% patients had severe TBI, respectively. The age of patients was significantly associated with the severity of an injury with severe grade, more frequent with increasing age (P = 0.006). The mean age of patients with mild, moderate and severe grades of injury were 26, 33 and 35 years, respectively [Figure 2].

- Mean age in different grades (P = 0.006) (Error bars indicate standard deviation)
Based on admission CT scan, 16%, 23%, 18%, 25% and 18% of patients belonged to TCDB CT category 1,2,3,4 and 5, respectively. As age increased, there was a significant increase in TCDB CT category, with category 5 more common in elderly, compared with category 1 being more frequent in young patients (P < 0.001) [Figure 3].

- Mean age across TCDB CT category (P < 0.001)
Of the total 244 patients, 111 patients required surgical intervention as per the mass effect, noted in CT scans. Out of 184 adult patients, 98 (53%) patients required surgical intervention, compared to 13 (22%) out of the 60 patients who were 1 to 18 years old [Figure 4]. The difference was statistically significant with an odds ratio of 4.1 (P < 0.001).
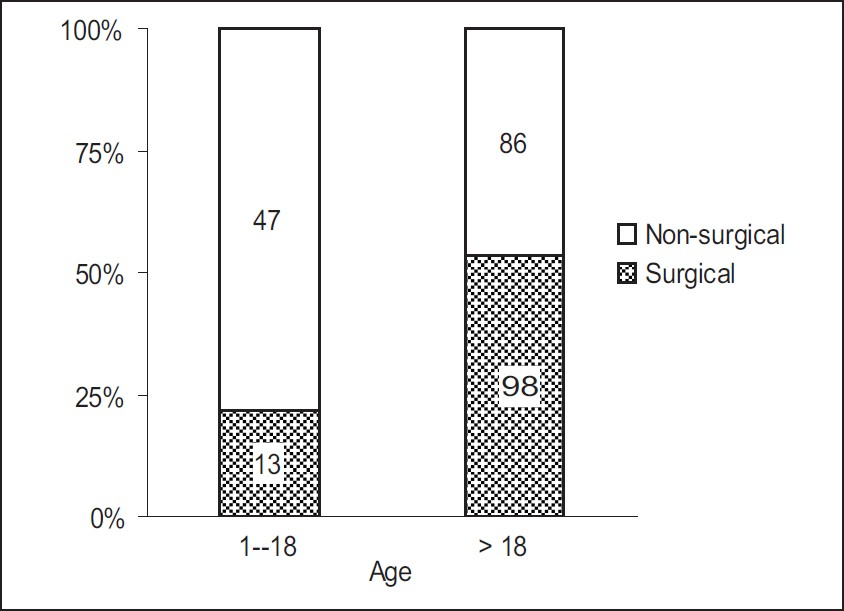
- Surgical intervention in different age groups (P < 0.001)
Mortality at 1 month was significantly associated with increasing age with 15%, 44% and 52% of patients dead at 1 month in the age groups < 18, 18 - 59 and > 59 years, respectively (P < 0.001) [Figure 5].

- Age vs mortality (P < 0.001)
Of the total 244 patients, the neurological outcome was assessed for 206 patients at 3 months and for 154 patients at 6 months, as the rest were lost to follow-up. As shown [Figure 6], the Glasgow Outcome Score at 6 months showed significant correlation with age, greater scores being more frequent in younger patients P < 0.001).

- Age vs glasgow outcome score (P < 0.001)
The outcome at 6 months characterized into favorable (good recovery and moderate disability), and unfavorable (severe disability, vegetative state and death) groups in various decades is as shown [Figure 7]. There was significantly better outcome in the 2nd decade as compared to the 1st decade. Moreover, unfavorable outcome showed significant association with an increasing age, every decade (P < 0.001).

- Decade wise outcome at 6 months (P < 0.001)
In multivariate analysis, after adjusting for the effect of GCS, TCDB CT category and surgical intervention using logistic regression, increasing age emerged as an independent risk factor for unfavorable outcome at 6 months. We analyzed the effect of various age thresholds individually on unfavorable outcome using logistic regression. The adjusted odds ratios for unfavorable outcome were 11.3, 53.3 and 1171 with respect to age thresholds 30, 40 and 50 years, respectively. Other age thresholds did not demonstrate statistical significance in multivariate analysis. The summary of independent effects of various age thresholds is as shown [Figure 8]. There is a stepwise, but significant increase in the probability of unfavorable outcome across the age groups centered on 40 years.
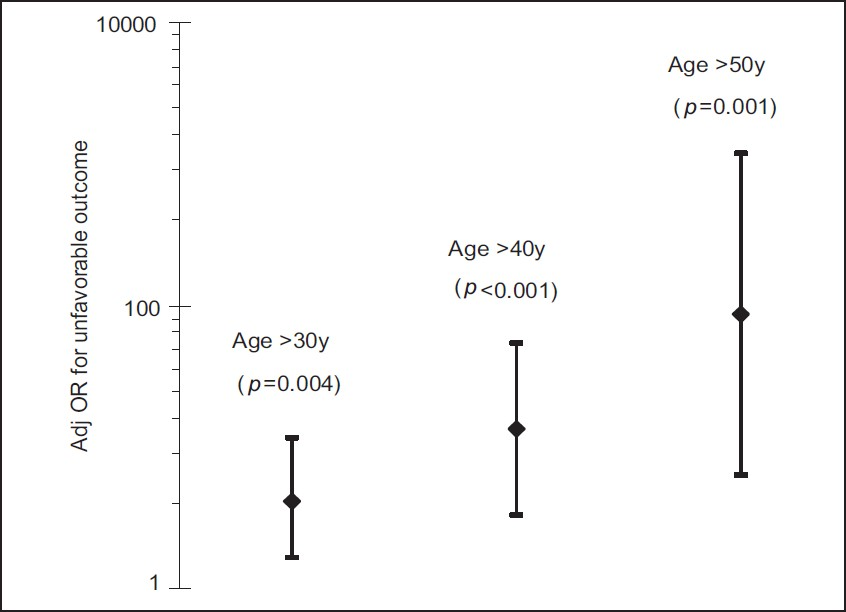
- Summary of significant age thresholds in multivariate analysis
The effect of age on outcome in subgroups based on GCS and surgical intervention is as shown [Table 1]. There was significant association of unfavorable outcome with age > 40 years in all the subgroups (P < 0.05).
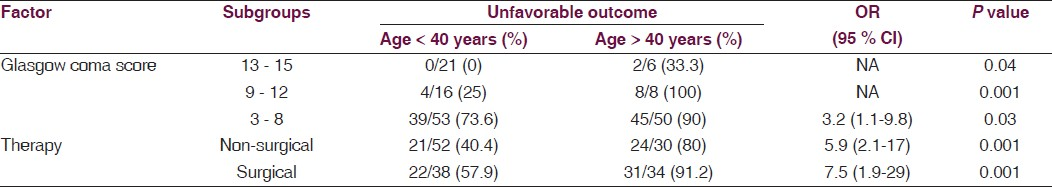
Discussion
TBI is a major health burden on our society.[12] An estimation of prognosis following TBI is still often described as ‘unduly optimistic, unnecessarily pessimistic or inappropriately ambiguous’.[7] As early as in 1970, Heiskanen et al[11] noted 78% mortality in patients over 60 years of age with severe TBI and recommended discretion before embarking on any special treatment. Since then, an increasing age has been noted in various studies to be associated with poorer outcome in patients with TBI.[3–6] However, it remains unclear how an association between patient age and outcome after TBI can be described best.[7] Several authors have noted varied age thresholds for poor prognosis. Gomez et al,[4] Bricolo et al,[12] Signorini et al[13] and Vollmer et al[14] noted age thresholds 35, 40, 50 and 55 years, respectively, while Braakman et al,[3] Heiskanen et al[11] and others[7] noted 60 years as the critical threshold. However, there are still many authors who noted the effect of age to be continuous[51516] and not discrete. Narayan et al[5] noted 57% and 78% unfavorable outcome in the age groups 41 - 60 and over 60 years, respectively. Our study noted stepwise worsening of outcome centered around 30 - 50 years with the best fitting threshold of 40 years in logistic regression, similar to the results of the meta-analysis, performed by Hukkelhoven et al.[17] Most of these studies were performed in severe TBI, whereas the present study includes patients with mild, moderate and severe TBI.
We also noted poorer outcome at 6 months among children < 10 years of age. This is in an agreement with Braakman et al,[3] Kriel et al,[18] and Raimondi et al,[19] while few others[2021] had noted better outcome in the 1st decade. However, the patients < 18 years of age had overall better outcome and lower mortality, compared with adults in our study, similar to previous other studies.[615]
The preponderance of falls, noted in the elderly in our study, has been observed in many previous studies.[14] Also, there was an age related trend towards increasing intracranial hematomas with the larger lesions observed more in older patients, similar to other studies.[7]
Our study had noted an impact of age of a person with TBI on poor outcome to be independent of the confounding effect of worsening TCDB CT category, diminishing GCS and surgical intervention. The limitations of this study are the relatively small number of patients in a single institution with inherent admission bias, and the crude outcome measure utilized.
The impact of age on outcome is probably due to the decreased capacity of adult brain for recovery as it ages, due to decreasing number of functioning neurons and greater exposure to subclinical insults.[20] The future area of research would be to explore the capacity for learning in the adult brain and how that is influenced by TBI. It would also be interesting to see how functional ability in everyday life is related to the age of patients with TBI.
Conclusions
In patients with TBI, an increasing age is significantly associated with unfavorable outcome at 6 months, in stepwise manner centered on a threshold of 40 years, independent of other prognostic factors.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Epidemiology of traumatic brain injuries: Indian scenario. Neurol Res. 2002;24:24-8.
- [Google Scholar]
- Systemic selection of prognostic features in patients with severe head injury. Neurosurg. 1980;6:362-70.
- [Google Scholar]
- Improved confidence of outcome prediction in severe head injury in the clinical examination, multimodality evoked potentials, CT scanning, and intracranial pressure. J Neurosurg. 1981;54:751-62.
- [Google Scholar]
- Age, severity, and outcome of head injury. In: Grossman RG, Gildenberg PL, eds. Head injury: Basic and clinical aspects. New York: Raven Press; 1982. p. :213-20.
- [Google Scholar]
- The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. J Neurotrauma. 2000;17:555-81.
- [Google Scholar]
- Assessment of coma and impaired consciousness- a practical scale. Lancet. 1974;2:81-4.
- [Google Scholar]
- A new classification of head injury based on computerized tomography. J Neurosurg (suppl). 1991;75:S14-20.
- [Google Scholar]
- Assessment of outcome after severe brain damage: A practical scale. Lancet. 1975;1:480-4.
- [Google Scholar]
- Predicting survival using simple clinical variables; a case study in traumatic brain injury. J Neurol Neurosurg Psychiatry. 1999;60:20-5.
- [Google Scholar]
- Age and outcome following traumatic coma: why do older patients fare worse? J Neurosurg (suppl). 1991;75:S37-49.
- [Google Scholar]
- Outcome from head injury related to patient's age. A longitudinal prospective study of adult and pediatric head injury. J Neurosurg. 1988;68:409-16.
- [Google Scholar]
- Patient age and outcome following severe traumatic brain injury: An analysis of 5600 patients. J Neurosurg. 2003;99:666-73.
- [Google Scholar]
- Closed head injury: Comparison of children younger and older than 6 years of age. Pediatr Neurol. 1989;5:296-300.
- [Google Scholar]
- Head injury in the infant and toddler.Coma scoring and outcome scale. Child's Brain. 1984;11:12-35.
- [Google Scholar]
- Factors affecting the clinical course of patients with severe head injuries. Part 1: Influence of biological factors. Part 2: Significance of post-traumatic coma. J Neurosurg. 1968;29:242-51.
- [Google Scholar]
- Severe head injuries in children: Early prognosis and outcome. Childs Nerv Syst. 1985;1:158-72.
- [Google Scholar]






