Translate this page into:
Posterior fossa involvement in a recurrent gliosarcoma
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Gliosarcoma (GSM) is a WHO grade 4 tumor and a variant of glioblastoma multiforme with predilection for the temporal lobe. We record, perhaps the first case in literature, of a temporal lobe GSM with recurrence involving the posterior fossa. A 50-year-old man presented to us with headache, vomiting, and lethargy of relatively recent onset. Magnetic resonance imaging revealed a well-circumscribed lesion in the left temporal lobe for which left temporal craniotomy with radical excision of the tumor was performed. Histopathology was suggestive of GSM. He presented to us within a month of the first surgery with a large recurrence involving the temporal lobe. He underwent a second surgery with radical excision of the tumor. Histopathology was confirmatory of GSM. He was administered concomitant chemotherapy and radiotherapy. Within a fortnight of starting adjuvant therapy, the bone flap started bulging and a repeat computed tomography scan revealed a large recurrence extending into the posterior fossa. The patient's relatives refused consent for third surgery and he finally succumbed on postoperative day 21. GSMs are aggressive tumors that have a temporal lobe predilection, but they may present anywhere in the brain. Detailed studies on larger cohort of cases are needed to understand the true nature of these biphasic tumors.
Keywords
Gliosarcoma
posterior fossa tumor
recurrence in posterior fossa
Introduction
Gliosarcoma (GSM) is a primary tumor of the brain composed of neoplastic glial cells in association with spindle cell sarcomatous elements.[1] It was first described by Stroebe but gained recognition following detailed histological analyses by Feigen and colleagues.[1–3] The 2007 World Health Organization classification scheme places primary GSM as a grade 4 neoplasm and a variant of glioblastoma multiforme (GBM).[4] GSMs have a temporal lobe predilection and ours is the first case describing a recurrence involving the posterior fossa.
Case Report
A 50-year-old man, working as a laborer presented to us with headache, occasional vomiting, loss of appetite, and lethargy since 15–20 days. Examination revealed loss of affect and impaired short-term memory. Magnetic resonance imaging (MRI) revealed a well-circumscribed lesion in the left temporal lobe, which was isointense on T1-weighted images (T1WI), hypointense on T2WI with postcontrast enhancement [Figure 1].
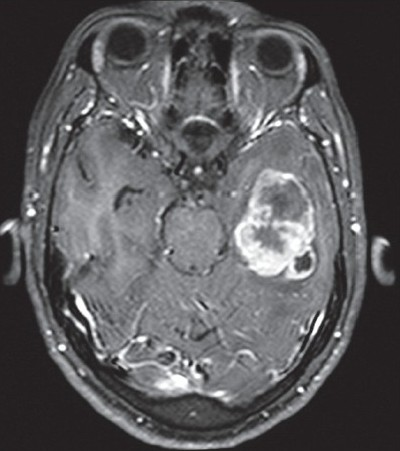
- Postcontrast axial MRI showing a large intra-axial space occupying lesion in the left temporal lobe with peripheral enhancement and peritumoral edema
The patient was operated; left temporal craniotomy with radical decompression of tumor was performed. The tumor was well circumscribed with a good plane of cleavage from the surrounding normal brain parenchyma. Intraoperative frozen was suggestive of high-grade neoplasm. Postoperatively, the patient had no added deficits, improved in cognition, was more alert, and was relieved of headache. Histopathology of the lesion was suggestive of GSM. He was advised radiotherapy and immunohistochemistry for further characterization of the tumor, but he was lost to follow-up and postoperative imaging also could not be done.
He presented to us 1 month later with complaints of headache, drowsiness, and decreased verbalization. On admission, the Glasgow Coma Scale was 14/15 with eye opening on command. A cranial computed tomography (CT) scan revealed a large tumor recurrence in the left temporal lobe with mass effect. The lesion was isodense on plain images with postcontrast enhancement [Figure 2].

- Postcontrast axial CT scan showing a large recurrent tumor almost occupying the whole posterior part of the left temporal lobe
He underwent emergency radical decompression of the tumor by reopening previous craniotomy site. Similar to the previous surgery, the tumor was well circumscribed, firm in consistency, and moderately vascular. The patient was extubated on table, had no added deficits, was more alert and improved in speech output. Postoperative CT showed residual tumor enhancement along the tentorial leaflet [Figure 3].
- Postoperative contrast axial CT showing radical excision of tumor with enhancement along the tentorial leaflet
Histologically, the tumor showed heterogenous morphology with areas composed of polygonal to irregular-shaped tumor cells with moderate to abundant eosinophilc cytoplasm in a background of neurofibrillary matrix [Figure 4b and c] admixed with spindle cell areas [Figure 4a and d]. A special stain for reticulin [Figure 5a] showed focal presence of intratumoral fine to coarse reticulin fibers with areas of pericellular architecture. On immunohistochemistry, the tumor was focally positive for glial fibrillary acidic protein [GFAP; Figure 5b] in the regions of polygonal tumor cell areas and reticulin-poor areas. It was diffusely positive for p53 protein [Figure 5c] and showed high MIB-1 labeling index of 15%–20% [Figure 5d]. As compared to histopathology of previous specimen, large zones of undifferentiated clonal tumor with higher mitotic activity was seen.
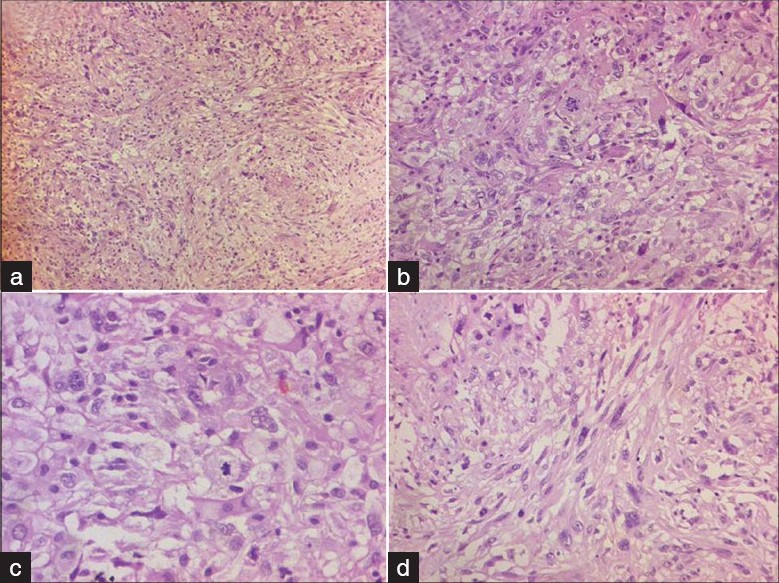
- Photomicrographs show a high-grade tumor composed of polygonal tumor cells (b and c) admixed with spindle cells (a and d). Nuclear pleomorphism and mitoses seen. (H and E, a and b: ×100; c and d: ×200)
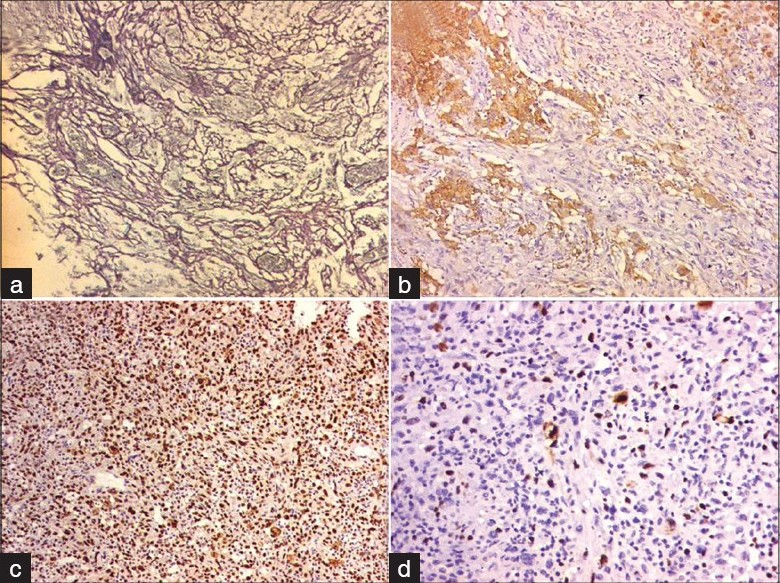
- (a) Photomicrograph shows increase in intratumoral reticulin (reticulin stain, ×100); (b) IHC; GFAP ×100); (c) (IHC; p53 ×100); and (d) IHC; MIB-1 ×200): Photomicrographs of the immunohistochemistry, which is focal positive for glial fibrillary acidic protein (b), which is retic poor areas and suggest glial area and the tumor is diffusely positive for p53 protein (c). MIB-1 labeling index (d) is approximately 8%–10%
The patient received combined radiotherapy (RT) and chemotherapy. He was given 60 Gy of radiation in fractionated doses with concomitant temozolamide.
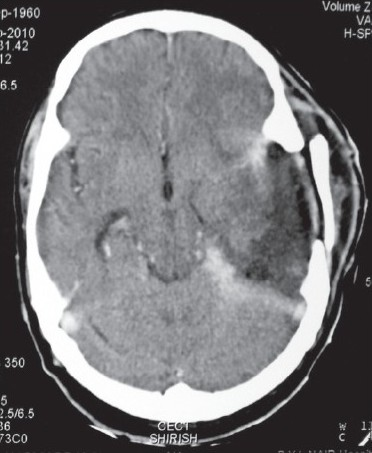
However, within a fortnight the bone flap started bulging and he became progressively drowsy. Repeat CT showed a large recurrence extending into the posterior fossa [Figure 6]. The lesion was isodense on plain scan with postcontrast enhancement. He was advised surgical decompression but relatives refused any operative intervention. He expired on day 21 of the second surgery.

- Postcontrast axial CT scan showing recurrent tumor in the left posterior temporal lobe extending into the posterior fossa and compressing the brain stem
Discussion
GSMs are rare and have an incidence of 1.8%–2.8% of that of GBMs.[56] The diagnosis of GSM should be made when a tumor contains an admixture of two distinctive neoplastic tissues. One is an anaplastic astrocytoma and the other resembles a fibrosarcoma.[37] Certain authors have suggested the presence of at least one atypical mesenchymal cell proliferation in one confluent medium power (10× objective with 10× eyepiece) field for a diagnosis of GSM.[8] Atypical components, such as osteochondroma or osteosarcoma have rarely been described in GSM.[910]
There are two theories proposed to explain the pathogenesis of GSM. The earlier theory suggested that the sarcomatous component originates from neoplastic transformation of hyperplastic blood vessels found in high-grade glioma.[2] The recent theory suggests monoclonal origin of both components of GSM with sarcomatous component originating via aberrant mesenchymal differentiation of the malignant glioma. This explains the absence of significant difference in clinical outcome between glioblastoma and GSM.[11] GSM has also been described following RT.[81213]
Although several studies have not shown any significant differences between GSM and GBM with regard to age, sex, size, clinical presentation, and median survival, they are established as two different pathologies.[3111415] Some studies showed a temporal lobe predilection for GSM, whereas others found no such difference.[3681115] A few studies have also found that GSM tends to be smaller at presentation as compared to GBM.[8] Despite these similarities, the two tumors differ in their intraoperative appearance (GSM being similar to meningioma due to its well-circumscribed nature), tendency for extraneural metastasis, and infrequency of epidermal growth factor receptor (EGFR) mutations.[31115]
GSM most commonly affects adults in the sixth or seventh decade of life and more commonly men (male:female ratio of 1.4 to 1.8:1).[515] The presenting signs and symptoms are consistent with those of an expanding space occupying lesion, such as headache, hemiparesis, seizures, and cognitive decline.[11] Table 1 shows clinical data of previously reported large series of GSMs.
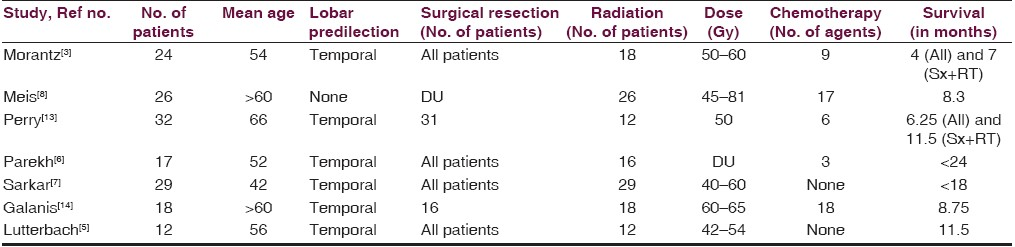
Treatment modalities for GSM include tumor resection, postoperative RT and chemotherapy[11] The median survival of GSM patients has been reported to be 9 months but there are some variables that alter this.[15] Patients diagnosed prior to 50 years had a higher median survival period of 15 months as compared to 7 months for those diagnosed after age of 50 years. Radical excision prolongs survival to 7–11 months as compared to 4 months with biopsy alone. Radiotherapy increased the survival rate from 4 to 10 months.[15] The role of chemotherapy is still uncertain with a few encouraging reports.[11]
Radiographic features of GSM include large necrotic areas with heterogeneous contrast enhancement in CT, whereas MRI typically shows marked peritumoral edema.[11] GSMs have a propensity for metastasis to other organs, most commonly lung and liver.[11] In spite of generous data being available on metastasis, there is very little data on contiguous recurrence of GSM postoperatively. We could find only two documented cases of recurrence. In the first case, recurrence of sarcomatous component in temporal lobe occurred following boron neutron capture therapy and in the second, recurrence occurred in the infratemporal fossa postoperatively. In the latter case, the authors have reported the presence of dural invasion and hence they could not excise the tumor radically .[1116] Ours is the first to report postoperative recurrence in the posterior fossa.
Conclusion
GSMs are aggressive tumors with a propensity for early recurrence. Although they have been reported to have a temporal lobe predilection, they can recur and regrow in any direction. Larger studies are needed to understand the true nature of these biphasic tumors.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Uber Entstehung und Bau der Gehirngliome. Beitr Pathol Anat Allg Pathol. 1895;18:405-86.
- [Google Scholar]
- Clinical and pathological study of 24 cases of gliosarcoma. J Neurosurg. 1976;45:398-408.
- [Google Scholar]
- The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97-109.
- [Google Scholar]
- A clinicopathological study of 29 cases of gliosarcoma with special reference to two unique variants. Indian J Med Res. 1997;106:229-35.
- [Google Scholar]
- Mixed glioblastoma multiforme and sarcoma: A clinicopathologic study of 26 radiation therapy oncology group cases. Cancer. 1991;67:2342-9.
- [Google Scholar]
- A clinical and pathological study of gliosarcoma. Zhonghua Bing Li Xue Za Zhi. 1996;25:129-31.
- [Google Scholar]
- Primary gliosarcoma: Key clinical and pathologic distinctions from glioblastoma with implications as a unique oncologic entity. J Neurooncol. 2010;96:313-20.
- [Google Scholar]
- Two autopsied cases of radiation-induced gliosarcoma. No Shinkei Geka. 1991;19:285-90.
- [Google Scholar]
- Clinicopathologic features of primary and postirradiation cerebral gliosarcoma. Cancer. 1995;75:2910-8.
- [Google Scholar]
- Clinical outcome of gliosarcoma compared with glioblastoma multiforme. North central cancer treatment group results. J Neurosurg. 1998;89:425-30.
- [Google Scholar]
- Adult gliosarcoma: Epidemiology, natural history and factors associated with outcome. Neuro-Oncology. 2009;11:183-91.
- [Google Scholar]
- Temporal gliosarcoma with extraneural metastasis. Neurol Med Chir (Tokyo). 2010;50:343-5.
- [Google Scholar]






