Translate this page into:
Role of nitrosative and oxidative stress in neuropathy in patients with type 2 diabetes mellitus
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
Evidences of oxidative and/or nitrosative stress in type 2 diabetes mellitus were demonstrated in experimental and human studies. This study is aimed to assess the serum peroxynitrite and oxidized lipoproteins in patients with type 2 diabetes mellitus presented with clinical and laboratory evidences of peripheral neuropathy.
Materials and Methods:
Eighty four patients with type 2 diabetes mellitus (51 of them had neuropathy) and 31 apparent healthy subjects were studied in the unit of neurophysiology at the University Hospital of Medical College, Al-Nahrin University in Baghdad, Iraq. Neuropathy total symptom score (NTSS), neuropathy impairment score in the lower leg (NIS-LL), and nerve conduction velocity of sensory (ulnar and sural) and motor (ulnar and common peroneal) nerves were used to assess the neuropathy. Fasting venous blood was obtained from each participant for the determination of serum glucose and oxidized lipoproteins.
Results:
The electrophysiology study revealed significant decrease in conduction velocity of ulnar (sensory and motor components), sural, and common peroneal nerves in diabetic neuropathy compared to diabetics without neuropathy and healthy subjects. Significant high level of serum peroxynitrite was found in diabetic patients with or without neuropathy compared with non-diabetics. The changes in serum-oxidized lipoproteins in patients with diabetics with or without neuropathy were non-significantly differed from healthy subjects. Neither nitrosative stress nor oxidative stress indices correlated with the variables that are related to the neuropathy.
Conclusion:
It concludes that evidence of nitrosative and to less extent the oxidative stress is associated with neuropathy in type 2 diabetes mellitus and their indices not correlated with variables related to neuropathy.
Keywords
Neuropathy
peroxynitrite
type 2 diabetes
Introduction
Peroxynitrite (ONOO•) is formed in vivo under oxidative stress in a diffusion-controlled reaction between nitric oxide (NO•) and superoxide anion (O2•-). ONOO• is a strong oxidizing and nitrating agent, which is involved in the development of a variety of pathological conditions including diabetes, cardiovascular, and neurodegenerative disorders. It plays a key role in the pathogenesis of the cardiovascular complications of diabetes, which underlie the development and progression of diabetic retinopathy, neuropathy, and nephropathy.[1] An abnormal production of reactive oxygen species (ROS) and the subsequent decrease in vascular bioavailability of NO• have long been proposed to be the common cause of the endothelial dysfunction, resulting from diabetes mellitus.[2] In experimental animal model of diabetes, ONOO• can suppress endothelial NO• synthase (eNOS) expression and decrease NO• levels.[3] In diabetic retinopathy, ONOO• impaired the production and activity of matrix metalloproteinase-7 (MMP-7), which cleaves pro-nerve growth factor (pro-NGF) extracellularly, leading to accumulation of pro-NGF and reducing NGF in samples from diabetic retinopathy patients and experimental models.[4] In diabetic nephropathy, excess ONOO• was generated from iNOS and play a key role in glomerular lesions.[56] Also, it could induce entire mitochondrial protein nitration and thereby it is responsible for the damage of renal mitochondria in diabetes.[7] Diabetic neuropathy, a major complication of diabetes, affects more than 60% of diabetic patients. Recently, involvement of ONOO• has been postulated in diabetic neuropathy. Nitrosative stress plays a major role in diabetic neuropathy at least in experimental type 1 diabetes.[8] In experimental animal model, iNOS plays a key role in ONOO• injury to peripheral nerve (particularly in axons and Schwann cells rather than dorsal root ganglion) in causing peripheral nerve dysfunction and degeneration.[9] Diabetic animals showed a significant decrease in motor nerve conduction velocity, nerve blood flow and nociception in terms of hyperalgesia, mechanical allodynia along with elevation in ONOO•.[10] And these functional and biochemical deficits are improved with using ONOO• decomposition catalysts.[10] At the molecular level, ONOO• -induced diabetic neuropathy via damaging DNA and over-activation of poly(ADP-ribose) polymerase (PARP), a nuclear enzyme activated after sensing DNA damage.[11] This study is aimed to assess the serum ONOO• and oxidized lipoproteins in patients with type 2 diabetes mellitus presented with clinical and laboratory evidences of neuropathy.
Materials and Methods
This study is conducted in the unit of neurophysiology, the University Hospital of Medical College, Al-Nahrin University in Baghdad, Iraq, over a period of two years. The study is approved by the local scientific committee of Medical College, Al-Nahrin University. A consent form was obtained from each participant prior to the study. A total number of 84 patients (30 male and 54 female) with history of type 2 diabetes mellitus; 51 of them (16 male and 35 female) presented with subjective symptoms of peripheral neuropathy and 33 of them (14 male and 19 female) without neuropathy as well as 31 (12 male and 19 female) healthy subjects served as control are enrolled in the study. Each patient and subject is clinically examined and the severity as well as the functional impairment were assessed using neuropathy total symptom score (NTSS)[12] and neuropathy impairment score in the lower leg (NIS-LL).[13] NTSS is a questionnaire that measures the frequency and intensity of numbness, pickling, aching pain, burning pain, lancinating pain and allodynia. In this measure, the symptom frequency was graded to occasional, frequent, and continuous while the symptom intensity was graded to absent, slight, moderate, and severe. Therefore, the score ranged between 0 (absent symptom) and 3.66 (severe continuous symptom). The neuropathy impairment score in lower limbs has 14 items that evaluates the muscle power, reflexes, and sensory modalities. The total score ranged between 0 and 88 points. The age factor is considered in scoring the reflexes using NTSS. Nerve conduction velocity was measured using DANTEC counterpoint clinical four channel electromyography system.
A fasting venous blood samples were obtained from participants and the sera were separated for determination of glucose, ONOO• and lipid peroxides.
Determination of ONOO•
Assessment of nitrosative stress status in patients was achieved by determination of ONOO•. ONOO•-mediated nitration of phenol was measured as described by van Uffelen et al.[14] Ten microliter of serum was added to 5 mM phenol in 50 mM sodium phosphate buffer (pH 7.4) to get a final volume of 2 mL. After 2 hours incubation in dark place at 37°C, 15 μL of 0.1M NaOH was added and the absorbance, at wavelength of 412 nm, of the samples were immediately recorded. The yield of nitrophenol was calculated from ε = 4400/M/cm.
Determination of lipid peroxides
Assessment of oxidative stress status of patients was achieved by determination of the thiobarbituric acid reactive substances in the fractionated lipoproteins.[15] Three milliliter serum was mixed with 100 μL sodium heparinate, 150 μL manganese chloride (10%), briefly vortex the mixture, then incubated for 10 min at room temperature after that centrifugation at 15000 rpm for 15 min. The pellet was separated from the supernatant and suspended in 3 mL sodium chloride (0.9%). The main constituents in the supernatant and pellet are high-density lipoprotein (HDL) and low-density lipoprotein (LDL), respectively. Thiobarbituric reactive substances were assayed in supernatant (which represented oxidized-HDL) and pellet (which represented oxidized-LDL). In brief, mix 0.3 mL of supernatant or reconstituted pellet with 1 mL (0.67% v/v) freshly prepared thiobarbituric acid, heat the mixture in water bath (95°C) for 30 min, cool the mixture immediately, then add 4 mL of methanol/n-butanol (3:17) mixture and spin at 2500 rpm at below the room temperature. The absorbance of upper (butanol) layer was recorded at wavelength of 535 nm and the concentrations of oxidized lipids were calculated from ε = 1.56 × 105/M/cm.
Statistical analysis
The results are expressed as number, percent, odd ratio and mean ± SE. The data were analyzed using two-tailed unpaired student's “t” test, difference between percentage test and simple correlation test taking P ≤ 0.05 as the lowest limit of significance.
Results
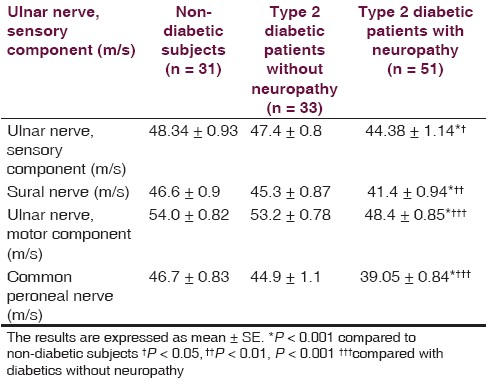
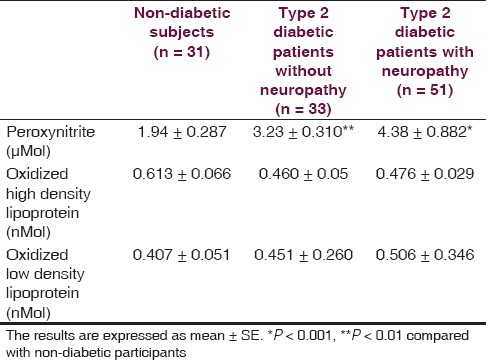
There is non-significant difference between non-diabetic (52.4 ± 2.0 year) and diabetic (54.4 ± 1.3 year with neuropathy, 52.9 ± 2.2 year without neuropathy) groups regarding the age. Fasting serum glucose and duration of illness of diabetic patients at the time of the study were ranged from 115 to 360 mg/dL and 3 months to 23 years, respectively. Forty six out of 51 diabetics with neuropathy scored TSS (maximum score was 11.32) compared to 5 out of 33 diabetics without neuropathy (maximum score was 4.55) and 5 out of 31 healthy individuals (maximum score was 4.66), and 39 out of 51 diabetics with neuropathy scored NIS-LL (maximum score was 28) compared to 4 out 33 diabetics without neuropathy (maximum score was 12.5) and 3 out of 31 healthy individuals (maximum score was 9.7). The nerve conduction velocities (m/s) of sensory component of ulnar nerve and sural nerve of diabetics with neuropathy were significantly lower than corresponding diabetics without neuropathy and non-diabetic subjects [Table 1]. The conduction velocities (m/s) of motor component of ulnar nerve and common peroneal nerve were also significantly less than corresponding diabetics without neuropathy and non-diabetic subjects [Table 1]. Table 2 shows that nitrosative stress is significantly existed in diabetic patients in terms of significant high serum ONOO•. Although the oxidized-LDL level is higher in diabetics than non-diabetic subjects, it does not reach to the level of significance. The oxidized-HDL level of diabetics is none significantly less than corresponding non-diabetic subjects, which achieved 78% [Table 2]. Oxidized lipoproteins as well as ONOO• do not correlate with duration of diabetes, serum glucose level, and the evidences of neuropathy in terms of TSS score, NIS-LL score, and conduction velocity in diabetics with or without neuropathy. The correlation factor varied between -0.252 (the relationship between oxidized-LDL and conduction velocity of motor component of ulnar nerve in diabetics with neuropathy) and 0.197 (the relationship between oxidized-LDL and fasting serum glucose in diabetics with neuropathy).
Discussion
The results reported in this study show that both oxidative and nitrosative stress are associated with diabetic neuropathy and to be significant with nitrosative stress. Previous studies reported significant high levels of lipid peroxides in sera of diabetic patients but not in fractionated lipoprotein in presence or absence of diabetic complications, which explain the non-significant high level of oxidized-LDL and low level of oxidized-HDL that observed in this study.[16] On the other hand, Migdalis et al,[17] found that serum thiobarbituric reacting substances in type 2 diabetes mellitus with neuropathy was significantly less than control level or diabetics without neuropathy. In experimental animal model using mice, oxidized low-density lipoproteins play a role in the development of neuropathy via a mechanism related to oxidative stress.[18] In human, Willems et al found the activity of lipid peroxidation did not increase in type 1 diabetic patients even with subclinical complications.[19]
There is no doubt that the availability of NO• is decreased in type 2 diabetic patients and the results of this study add a further finding of significant high level of peroxynitrite (ONOO•), an end-product of non-enzymatic reaction between nitric oxide (NO•) and superoxide anion (O2•-) radicals.[20–22] The non-significant correlation between oxidative or nitrosative stress with nerve conduction velocity could be attributed to the wide variation in duration of illness, small sample size of patient, and the levels of lipoprotein oxides and ONOO• in biological fluids rather than in nerve tissue. In the non-diabetic experimental animal model the significant increase lipid peroxides in nerve is correlated with electrophysiological nerve indices.[23] Moreover, in diabetic experimental animal model, the plasma rather than the neural lipid peroxides are significantly increased and the duration of hyperglycemia is an important factor in determining the lipid peroxides level.[24] Therefore, the changes in the oxidized lipoproteins and the significant high ONOO• level is just an association with diabetic neuropathy rather than inducing factors and further comparative study between type 2 diabetics with and without neuropathy is recommended. It concludes that evidence of nitrosative and to less extent the oxidative stress is associated with neuropathy in type 2 diabetes mellitus and their indices not correlated with variables related to neuropathy.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Role of peroxynitrite in the pathogenesis of cardiovascular complications of diabetes. Curr Opin Pharmacol. 2006;6:136-41.
- [Google Scholar]
- Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. 2010;22:2741-8.
- [Google Scholar]
- Peroxynitrite mediates diabetes-induced endothelial dysfunction: Possible role of rho kinase activation. Exp Diabetes Res. 2010;2010:247861.
- [Google Scholar]
- Diabetes-induced peroxynitrite impairs the balance of pro-nerve growth factor and nerve growth factor, and causes neurovascular injury. Diabetologia. 2011;54:657-68.
- [Google Scholar]
- Glycoxidative and nitrosative stress in kidney of experimental diabetic rats: Effects of the prydoindole antioxidant stobadine. Neuro Endocrinol Lett. 2010;31:313-8.
- [Google Scholar]
- Peroxynitrite plays a key role in glomerular lesions in diabetic rats. J Nephrol. 2009;22:800-8.
- [Google Scholar]
- Peroxynitrite-induced protein nitration is responsible for renal mitochondrial damage in diabetic rat. J Endocrinol Invest. 2010;33:140-6.
- [Google Scholar]
- Role for nitrosative stress in diabetic neuropathy: Evidence from studies with a peroxynitrite decomposition catalyst. FASEB J. 2005;19:401-3.
- [Google Scholar]
- Inducible nitric oxide synthase gene deficiency counteracts multiple manifestations of peripheral neuropathy in a streptozotocin-induced mouse model of diabetes. Diabetologia. 2008;51:2126-33.
- [Google Scholar]
- Amelioration of neurological and biochemical deficits by peroxynitrite decomposition catalysts in experimental diabetic neuropathy. Eur J Pharmacol. 2008;596:77-83.
- [Google Scholar]
- Concurrent targeting of nitrosative stress-PARP pathway corrects functional, behavioral and biochemical deficits in experimental diabetic neuropathy. Biochem Biophys Res Commun. 2010;391:102-6.
- [Google Scholar]
- Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multicentre randomized controlled trial (ALADIN Study) Diabetologia. 1995;38:1425-33.
- [Google Scholar]
- Variables influencing neuropathic endpoints: The Rochester Diabetic Neuropathy Study of Healthy Subjects. Neurology. 1995;45:1115-21.
- [Google Scholar]
- Intracellular but not extracellular conversion of nitroxyl anion into nitric oxide leads to stimulation of human neutrophil migration. Biochem J. 1998;330:719-22.
- [Google Scholar]
- Premature peripheral vascular disease: Clinical profile and abnormal lipid peroxidation. Cardiovasc Surg. 1998;6:188-93.
- [Google Scholar]
- High levels of plasma malondialdehyde, protein Carbonyl, and fibrinogen have prognostic potential to predict poor outcomes in patients with diabetic foot wounds: A preliminary communication. Int J Low Extrem Wounds. 2008;7:198-203.
- [Google Scholar]
- Lipid peroxides in type 2 diabetic patients with neuropathy. Res Commun Mol Pathol Pharmacol. 2005;117-118:5-12.
- [Google Scholar]
- Dyslipidemia-induced neuropathy in mice: The role of oxLDL/LOX-1. Diabetes. 2009;58:2376-85.
- [Google Scholar]
- Serum antioxidant status and oxidized LDL in well-controlled young type 1 diabetic patients with and without subclinical complications. Atherosclerosis. 1998;137(Suppl):S61-4.
- [Google Scholar]
- Participation of neuronal nitric oxide synthase in experimental neuropathic pain induced by sciatic nerve transection. Braz J Med Biol Res. 2010;43:367-76.
- [Google Scholar]
- Concurrent targeting of nitrosative stress-PARP pathway corrects functional, behavioral and biochemical deficits in experimental diabetic neuropathy. Biochem Biophys Res Commun. 2010;391:102-6.
- [Google Scholar]
- Peripheral neuropathy in mice with neuronal nitric oxide synthase gene deficiency. Int J Mol Med. 2009;23:571-80.
- [Google Scholar]
- Effects of acrylamide on the nervous tissue antioxidant system and sciatic nerve electrophysiology in the rat. Neurochem Res. 2008;33:2310-7.
- [Google Scholar]
- Nerve conduction and antioxidant levels in experimentally diabetic rats: Effects of streptozotocin dose and diabetes duration. Metabolism. 1999;48:442-7.
- [Google Scholar]






