Translate this page into:
Comparison of antinociceptive effect of the antiepileptic drug gabapentin to that of various dosage combinations of gabapentin with lamotrigine and topiramate in mice and rats
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Newer anticonvulsants have a neuromodulatory effect on pain perception mechanisms in a hyperexcitable and damaged nervous system.
Aim:
This study was designed to study the analgesic effects of gabapentin alone and in combination with lamotrigine and topiramate in experimental pain models.
Materials and Methods:
Adult albino mice (n=490) weighing 20–30 g and rats (n=130) weighing 100–200 g were injected intraperitoneally with gabapentin, lamotrigine, and topiramate alone and in different dose combinations. The hot-plate method, tail-flick method, capsaicin-induced mechanical hyperalgesia, and formalin assay were used to assess the antinociceptive effects.
Results:
Of the three antiepileptic drugs, when given separately, gabapentin was more efficacious than either topiramate or lamotrigine in all the pain models. Combination of 25 mg/kg gabapentin with 25 mg/kg topiramate was more efficacious (P<.05) than 50 mg/kg gabapentin alone in the capsaicin-induced mechanical hyperalgesia test. Similarly, 50 mg/kg gabapentin with 50 mg/kg topiramate or 5 mg/kg lamotrigine was more efficacious (P<.05) than 50 or 100 mg/kg gabapentin alone in late-phase formalin-induced behaviors.
Conclusions:
Combination of gabapentin with either lamotrigine or topiramate produced better results than gabapentin alone in capsaicin-induced mechanical hyperalgesia test and in late-phase formalin-induced behaviors.
Keywords
Analgesia
formalin assay
gabapentin
lamotrigine
mechanical hyperalgesia
radiant heat
topiramate
thermal nociception
Introduction
Pain is a commonly experienced and feared symptom[1] in many diseases. Neuropathic pain affects millions of people around the world. Because of nonavailablity or inadequacy of treatment patients are often forced to experience symptoms, such as pain, paresthesia, dysesthesia, hyperalgesia, and allodynia for many years.[2] Pain caused by dysfunction or damage to the peripheral or central nervous system is typified by the symptoms described by patients with painful diabetic neuropathy, postherpetic neuralgia, central stroke pain, and trigeminal neuralgia.[3] Neuropathic pain occurs in one-third of cancer patients either alone or in combination with nociceptive pain.[4]
Failure to manage pain properly is due to several factors. In developing countries, these factors include geographical variation and limited resources, legal restrictions on import of drugs like morphine, lack of proper medical care, fear of drug addiction, drug tolerance, and side effects.[1] Neuropathic pain is characterized by both positive (hyperalgesia and allodynia) and negative (sensory deficit) symptoms that are unrelieved by many commonly used analgesics.[4]
Significant improvement of neuropathic pain on treatment with the with newer anticonvulsants has been reported, and studies have demonstrated the neuromodulatory effect of these drugs on a hyperexcitable damaged nervous system.[5] Antiepileptics are increasingly utilized in the treatment of neuropathic pain. This class of drugs works via three major mechanisms, i.e., potentiation of GABA transmission, reduction of glutamate-mediated excitatory transmission, and blockade of voltage-activated ion channels. The latter mechanism of action, in particular, is responsible for the success of the newer generation of antiepileptic drugs such as lamotrigine, gabapentin,[4] and topiramate, which have all been shown to be effective in animal models of neuropathic pain.[6] The newer agents have less potential for drug interactions and a more favorable side effect profile.[7]
With regard to neurological conditions other than epilepsy, experimental evidence for the efficacy of antiepileptic drugs is only available for the treatment of patients with trigeminal neuralgia, neuropathic pain syndromes, migraine, and essential tremor.[8] Recent studies indicate that peripheral neuropathic pain is generated through a focal inflammatory process rather than via axonal destruction. This process also appears to involve mRNA regulation of fast sodium channels, which produce ectopic discharge and are presumably responsible for pain generation.[9] Emerging evidence from animal models of neuropathic pain suggests that many pathological and biochemical changes occur in the peripheral and central nervous systems. Similarities between the pathologic phenomena observed in epilepsy models and in neuropathic pain models justify the use of anticonvulsant drugs in the symptomatic management of neuropathic pain. Positive results from laboratory and clinical trials further support such use.[10] Enhancement of GABA neurotransmission may provide an approach to diminish the level of nociception in various pain states. Several studies have implicated GABAA and GABAB receptors in the spinal nociceptive circuit.[11] GABAA, but not GABAB, receptor agonists inhibit NMDA-induced behaviors.[12] Several antiepileptic drugs have been shown to have either a direct or an indirect influence on GABAergic transmission in the brain and antiepileptic drugs, such as gabapentin, lamotrigine, and topiramate, are potential therapeutic agents for the management of chronic pain.[1113]
Gabapentin is a structural analogue of GABA whose mechanism of action is currently unknown. It lacks affinity for both GABAA and GABAB receptors and fails to inhibit GABA release[14] or reuptake.[15] It may, however, alter GABA metabolism, its nonsynaptic release, or its reuptake by GABA transporters. An increase in brain GABA concentration has been observed in humans experiencing pain. It is transported in the brain by the L-amino acid transporter.[16] Besides, it also binds to the α2δ subunit of the voltage-sensitive calcium channels.[16] Several studies have shown gabapentin to be antinociceptive.[11] Gabapentin reverses the hyperalgesia and allodynia observed after peripheral nerve injury[1718] and suppresses spontaneous ectopic discharge from peripheral nerves.[1920]
Lamotrigine is an inhibitor of the voltage-gated sodium channel and also inhibits the voltage-gated calcium channel.[16] Lamotrigine's inhibition of the voltage-gated sodium channel stabilizes the presynaptic neuronal membrane,[21] thus preventing the release of excitatory neurotransmitter[22] and inhibiting sustained repetitive neuronal firing.[23] These qualities of lamotrigine are suggestive of a drug having antinociceptive properties.[11]
Topiramate blocks repetitive firing of cultured spinal cord neurons, and its mechanism of action, therefore, is likely to involve blocking of voltage-dependent sodium channels. In addition, topiramate appears to potentiate the inhibitory effect of GABA and also depress the excitatory action of kainate on AMPA receptors.[16] Thus it may be useful in the treatment of a wide variety of neuropathic pain syndromes.[6]
Past studies have examined the antinociceptive effect of gabapentin, lamotrigine, and topiramate. However, to the best of our knowledge, no study has examined the efficacy of of gabapentin, lamotrigine, and topiramate, given separately as well as in different dosage combinations. Therefore, we designed this study to examine the antinociceptive capability of gabapentin when used alone and in combination with lamotrigine and topiramate. We used a broad range of nociceptive tests, including acute (hot-plate) through tonic (formalin assay) to chronic (mechanical hyperalgesia - Randall–Selitto test).
Materials and Methods
Drugs and chemicals
Gabapentin (Gabantin™, 500 mg) was purchased from Sun Pharmaceutical Industries, India; lamotrigine (Lametec DT™, 25 mg) from Cipla Ltd. Verna, Goa 403722, India; and topiramate (Topaz™, 100 mg) from Intas Pharmaceuticals, Selagmi, Dehradun 248197, India. Capsaicin (8-methyl-N-vanillyl-trans-6-nonenamide) was obtained from Fluka-Sigma-Aldrich, pf. D-89555, 07329/970 Steinheim, Switzerland; and Tween-80® (polyoxyethylene sorbitan monooleate) from Merck Ltd., Mumbai- 400 018, India.
Animals
Experiments were performed on adult albino mice (n=490) weighing 20–30 g and rats (n=130) weighing 100–200 g. The animals were produced in the laboratory breeding house of the Department of Pharmacology. The animals were maintained under controlled room temperature (25±2°C) and light-dark (12:12 hour) conditions and were given food pellets and water ad libitum. Before conducting the experiment, ethical clearance was obtained from the local Ethical Committee on Animal Research and the ethical guidelines for investigation of experimental pain in conscious animals were followed in accordance with IASP (International Association for the Study of Pain).[24] The animals were randomly divided into 13 groups of 10 animals each, as follows: group 1 (control, saline 10 mg/kg), group 2 (gabapentin 50 mg/kg), group 3 (gabapentin 100 mg/kg), group 4 (topiramate 50 mg/kg), group 5 (topiramate 100 mg/kg), group 6 (lamotrigine 10 mg/kg), group 7 (lamotrigine 50 mg/kg), group 8 (gabapentin 25 mg/kg and topiramate 25 mg/kg), group 9 (gabapentin 50 mg/kg and topiramate 50 mg/kg), group 10 (gabapentin 25 mg/kg and lamotrigine 5 mg/kg), group 11 (gabapentin 50 mg/kg and lamotrigine 25 mg/kg), group 12 (topiramate 25 mg/kg and lamotrigine 5 mg/kg), and group 13 (topiramate 50 mg/kg and lamotrigine 25 mg/kg).
Drug preparation
Drugs were administered i.p. in a volume of 10 ml/kg with a 27-gauge needle attached to the 1-ml disposable syringe. The drugs were diluted in 0.9% saline for all i.p. injections. Capsaicin was dissolved in a vehicle containing 7.5% Tween-80® in distilled water.[25] The Tween-80® /distilled water vehicle was used for the control injection. Intraplantar injection of the capsaicin was given in a volume of 10 μl using a microsyringe (Hamilton, Bonaduz, Switzerland) fitted with a 30-gauge needle. Rats received unilateral intradermal injection in the mid-plantar surface of the hind paw. The appearance of a bleb at the injection site indicated a successful injection.[26]
Experimental design
Hot-plate method
The thermal noxious stimulus was administered to mice by placing them on a hot-plate (Ugo Basile, Italy) maintained at 53°C for 10 min prior to the experiment. Drugs were injected i.p. 30 min before placing the mice on the hot-plate and the reaction time (hot-plate latency) was recorded. The reaction time was taken as the period between placing the mice on the hot-plate and the time when they jumped or licked their paws. A cutoff time of 60 sec was used to prevent any thermal injury to the mice.[1127]
Tail-flick method
Pain was induced by focusing infrared light on the tails of the mice (Tail-Flick Unit, Ugo Basile, Italy) 5 cm from the tip of the tail. Reaction time (tail-flick latency) was the interval between focusing of the infrared light on the tail and the withdrawal of the tail. A cutoff time of 30 sec was used.[27] The drugs were injected i.p. 30 min prior to the test.
Capsaicin-induced mechanical hyperalgesia
Intraplantar injection of capsaicin (10 μg), with 7.5% Tween-80® in distilled water as vehicle, was given in a volume of 10 μl. Mechanical nociceptive thresholds were assessed by applying an increasing noxious pressure stimulus to the distal portion of the plantar surface of the hind paw using the Analgesy-Meter® (Ugo Basile, Italy) according to the method of Randall and Selitto (1975). The site of stimulation was an area of the hind paw between the pads at the base of the third and fourth digits, distal to the site of capsaicin injection. Control (vehicle) or test drug was administered 30 min before capsaicin injection and paw withdrawal threshold was measured 15 min after the capsaicin injection. The cutoff weight was set at 500 g to prevent any tissue damage and the endpoint was taken as complete paw withdrawal.[26]
Formalin assay
The formalin test was used as a tonic model of nociception. Two phases of behavior follow injection of formalin (20 μl of 5% formalin) into the hind paw.[11] The first phase consists of intense licking and biting of the injected paw for the first 5 min followed by a period of little activity. The second phase spans from 15–30 min after the formalin injection and involves the period of licking and biting of the injected paw. The first phase is considered to be the model of acute chemical pain, whereas the second phase reflects a state of central sensitization. Mice were kept in clear plastic chambers individually at least 1 hour before the test and the amount of time spent on licking and biting the injected paw was recorded at 5 min intervals for 0–30 min after formalin injection.[11] Drugs were administered i.p. 15 min before the injection of formalin.
Statistical analysis
Both parametric (one-way ANOVA) and nonparametric (Kruskal-Wallis) statistical tests were used to analyze the data. Kruskal-Wallis nonparametric statistical analysis produced the same results as the analysis of variance. Only the parametric results were reported in the figures, and all data were expressed as the mean ± Standard error of the mean. Significance of difference between groups was further analyzed with Dunnett's t test for multiple post hoc comparisons. P<.05 was considered statistically significant at 95% confidence interval (CI).
Results
Role of antiepileptic drugs in acute thermal nociception (hot-plate test)
The effect of three antiepileptic drugs on acute thermal nociception was tested using the hot-plate test. Intraperitoneal administration of gabapentin (50 and 100 mg/kg) increased hot-plate latencies. Combination of gabapentin (25 and 50 mgkg) either with topiramate (25 and 50 mg/kg) or with lamotrigine (25 mg/kg) also significantly increased hot-plate latencies; however, 25 mg/kg gabapentin with 5 mg/kg lamotrigine had no effect on hot-plate latencies. No combination with gabapentin produced more superior results than the gabapentin alone at the doses tested in the experiment [Figure 1].

- Responses of different groups in the hot-plate test. Group 4 - topiramate 50 mg/kg; group 5 - topiramate 100 mg/kg; group 6 - lamotrigine 10 mg/kg; group 7 - lamotrigine 50 mg/kg; group 8 - gabapentin 25 mg/kg + topiramate 25 mg/kg; group 9 - gabapentin 50 mg/kg + topiramate 50 mg/kg; group 10 - gabapentin 25 mg/kg + lamotrigine 5 mg/kg; group 11 - gabapentin 50 mg/kg + lamotrigine 25 mg/kg; group 12 - topiramate 25 mg/kg + lamotrigine 5 mg/kg; group 13 - topiramate 50 mg/kg + lamotrigine 25 mg/kg. There were 10 mice per group. Values are mean ± standard error of the mean. P values: a<.05, b<.01 from saline. Data were analyzed by one-way ANOVA followed by Dunnett's t test, 95% confidence interval. GBP = gabapentin.
Role of antiepileptic drugs in radiant heat nociception (tail-flick test)
Administration of gabapentin at the doses of 50 and 100 mg/kg i.p. 30 min prior to the experiment did not produce any effect on tail-flick latency, but topiramate and lamotrigine dose-dependently increased the tail-flick latency though this was not significant when compared to control. Gabapentin (50 and 100 mg/kg in combination with either topiramate (50 and 100 mg/kg) or lamotrigine (5 and 25 mg/kg) increased tail-flick latency dose-dependently [Figure 2].
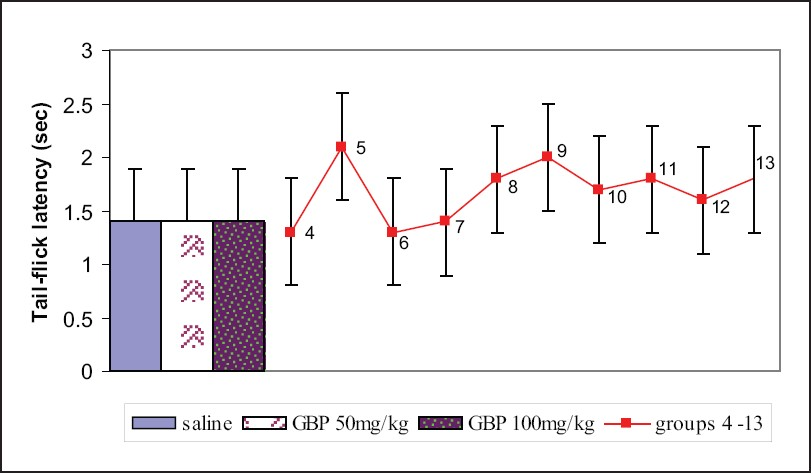
- Responses of different groups in the tail-flick test. Group 4 - topiramate 50 mg/kg; group 5 - topiramate 100 mg/kg; group 6 - lamotrigine 10 mg/kg; group 7 - lamotrigine 50 mg/kg; group 8 - gabapentin 25 mg/kg + topiramate 25 mg/kg; group 9 - gabapentin 50 mg/kg + topiramate 50 mg/kg; group 10 - gabapentin 25 mg/kg + lamotrigine 5 mg/kg; group 11 - gabapentin 50 mg/kg + lamotrigine 25 mg/kg; group 12 - topiramate 25 mg/kg + lamotrigine 5 mg/kg; group 13 - topiramate 50 mg/kg + lamotrigine 25 mg/kg; There were 10 mice per group. Values are mean ± standard error of the mean. Data were analyzed by one-way ANOVA followed by Dunnett's t test, 95% confidence interval. GBP = gabapentin.
Role of antiepileptic drugs in capsaicin-induced mechanical hyperalgesia (mechanical analgesymeter)
Administration of gabapentin at a dose of 100 mg/kg i.p. 30 min prior to intraplantar injection of capsaicin (10 μg) caused significant increase in the paw withdrawal threshold as compared to the control group. Gabapentin (25 and 50 mg/kg) with topiramate (25 and 50 mg/kg) also significantly increased the paw withdrawal threshold pressure as compared to the control and the gabapentin-only (50 mg/kg) groups. The combination of topiramate (25 and 50 mg/kg) with lamotrigine (5 and 25 mg/kg) produced significantly higher paw withdrawal thresholds than gabapentin at 50 mg/kg. Moreover, 25 mg/kg topiramate and 5 mg/kg lamotrigine in combination produced greater increase in paw withdrawal thresholds than did 100 mg/kg gabapentin [Figure 3].

- Responses of different groups in the mechanical analgesymeter. Group: 4 - topiramate 50 mg/kg; group 5 - topiramate 100 mg/kg; group 6 - lamotrigine 10 mg/kg; group 7 - lamotrigine 50 mg/kg; group 8 - gabapentin 25 mg/kg + topiramate 25 mg/kg; group 9 - gabapentin 50 mg/kg + topiramate 50 mg/kg; group 10 - gabapentin 25 mg/kg + lamotrigine 5 mg/kg; group 11 - gabapentin 50 mg/kg + lamotrigine 25 mg/kg; group 12 - topiramate 25 mg/kg + lamotrigine 5 mg/kg; group 13 - topiramate 50 mg/kg + lamotrigine 25 mg/kg. There were 10 mice per group. Values are mean ± standard error of the mean. P values: a<.05, b< .01 from saline; c<.05, d<.01 from GBP 50 mg/kg; e<.05 from GBP 100 mg/kg. Data were analyzed by one-way ANOVA followed by Dunnett's t test, 95% confidence interval. GBP = gabapentin.
Role of antiepileptic drugs in tonic nociception (formalin assay)
Intraperitoneal treatment with gabapentin, topiramate, and lamotrigine dose-dependently decreased the formalin-induced acute-phase and late-phase (central sensitization) behaviors. Gabapentin at 100 mg/kg significantly reduced both acute-phase and late-phase behaviors. Lamotrigine (10 and 50 mg/kg) produced significant results in the acute phase. Combination of gabapentin either with topiramate or with lamotrigine at the doses tested in the experiment had significant effects both in acute-phase and late-phase behaviors. Pretreatment with the combination of gabapentin 50 mg/kg and topiramate 50 mg/kg produced greater decrease in late-phase behavior than gabapentin alone at 100 mg/kg. Similar efficacy was seen with the combination of gabapentin 50 mg/kg and lamotrigine 25 mg/kg [Figures 4a and 4b].

- Responses of different groups in formalin assay. (a) Acute phase (0–5 min), (b) late phase (15–30 min). Group 4 - topiramate 50 mg/kg; group 5 - topiramate 100 mg/kg; group 6 - lamotrigine 10 mg/kg; group 7 - lamotrigine 50 mg/kg; group 8 - gabapentin 25 mg/kg + topiramate 25 mg/kg; group 9 - gabapentin 50 mg/kg + topiramate 50 mg/kg; group 10 - gabapentin 25 mg/kg + lamotrigine 5 mg/kg; group 11 - gabapentin 50 mg/kg + lamotrigine 25 mg/kg; group 12 - topiramate 25 mg/kg + lamotrigine 5 mg/kg; group 13 - topiramate 50 mg/kg + lamotrigine 25 mg/kg. There were 10 mice per group. Values are mean ± standard error of the mean. P values: a<.05, b<.01 from saline; c<.05 from GBP 50 mg/kg. Data were analyzed by one-way ANOVA followed by Dunnett's t test, 95% confidence interval. GBP = gabapentin.
Discussion
This study assessed the ability of three antiepileptic drugs gabapentin, topiramate, and lamotrigine, used alone and in various combinations with one other, to produce antinociception in four pain models. Gabapentin decreased the hot-plate responses at 50 mg/kg and 100 mg/kg. However, gabapentin had no effect in the tail-flick test, a finding that is consistent with previous studies.[111828] Pretreatment with gabapentin prevented the development of secondary mechanical hyperalgesia by intraplantar capsaicin injection; this is also consistent with earlier reports in the literature.[26] Studies have shown that gabapentin inhibits capsaicin-evoked nociceptive spinal transmission[29] and also suppresses cutaneous hyperalgesia following heat-capsaicin sensitization in healthy volunteers.[30] Mechanical hyperalgesia is mediated by a distinct population of neurons[31] and gabapentin has preferential action on these neurons.[26] In the formalin test, which is a model of acute chemical pain (acute phase) and tonic nociception involving central sensitization (late phase),[11] gabapentin at 50 mg/kg had no effect in acute-phase behaviors though it inhibited these behaviors at 100 mg/kg. However, gabapentin dose-dependently (at both 50 and 100 mg/kg) inhibited late-phase formalin-induced behaviors; this is consistent with previous reports.[32] Topiramate and lamotrigine when given separately inhibited responses in acute thermal nociception. However, these two antiepileptic drugs showed no significant effect in the tail-flick test, mechanical hyperalgesia, and formalin-induced late-phase behaviors though they had borderline statistical significance at the doses tested in the experiment. Lamotrigine at 10 mg/kg reduced the acute-phase behaviors in formalin assay. The weak effects of gabapentin, topiramate, and lamotrigine in acute thermal nociception and the early phase of formalin-induced pain are consistent with previous reports that the efficacy of these antiepileptic drugs in models of acute nociception is very low.[113233]
The results of the present study suggest that antiepileptic drugs have little or no effect on most measures of normal transient nociceptive signaling but, rather, inhibit sensitized signaling associated with allodynia and hyperalgesia.[11] This interpretation is supported by the fact that gabapentin has no effect on normal afferent fiber activity, but inhibits the ectopic discharge activity associated with peripheral nerve injury.[20]
Gabapentin in combination with topiramate or lamotrigine at different doses was not more efficacious in acute thermal nociception and radiant-heat nociception than gabapentin alone. In capsaicin-induced mechanical hyperalgesia, 25 mg/kg gabapentin with 25 mg/kg topiramate had more efficacy than 50 mg/kg gabapentin. Combination of 50 mg/kg gabapentin either with 50 mg/kg topiramate or with 5 mg/kg lamotrigine was more efficacious than gabapentin alone in decreasing late-phase formalin-induced behaviors. A combination of drugs probably targets different sites and receptors[34] to produce better efficacy. However, before clear therapeutic recommendations can be made, further research is needed to ensure that the outcomes are reproducible. Transmission of painful stimuli through the spinal cord and central nervous system is modulated by excitatory and inhibitory neurotransmitters, as well as action at sodium and calcium channels. Glutamate is the most important excitatory neurotransmitter, while the most important inhibitory neurotransmitter is GABA. Antiepileptic drugs are thought to relieve neuropathic pain through interaction with specific neurotransmitters and ion channels, with inhibition of neuronal activities.[35] These drugs act at several sites that may be relevant to pain, but the precise mechanism of their analgesic effect remains unclear.[36] These drugs are thought to limit neuronal excitation and enhance inhibition. Relevant sites of action include voltage-gated ion channels (sodium and calcium), ligand-gated ion channels, the excitatory receptors of glutamate and NMDA, and the inhibitory receptors for GABA and glycine.[37]
In conclusion, this study demonstrates the effects of gabapentin and the combination of gabapentin with either topiramate or lamotrigine at different doses in the hot-plate test, the tail-flick test, the capsaicin-induced mechanical hyperalgesia model of neuropathic pain, and formalin assay. To the best of our knowledge, this study is the first to compare gabapentin and the combination of gabapentin with topiramate or lamotrigine in the above-mentioned models of nociception. Gabapentin was more efficacious than either topiramate or lamotrigine in all the pain models when the drugs were given separately. The combination of 25 mg/kg gabapentin with 25 mg/kg topiramate was more efficacious than 50 mg/kg gabapentin alone in the capsaicin-induced mechanical hyperalgesia test. Similarly, 50 mg/kg gabapentin with 50 mg/kg topiramate or 5 mg/kg lamotrigine was more efficacious than 50 or 100 mg/kg gabapentin alone in late-phase formalin-induced behaviors. However, further study is required to explain the mechanisms involved in producing this difference in efficacies of these antiepileptic drugs and to elucidate the potential therapeutic utility of such combinations in neuropathic pain.
We thank Ms Rusha Tamrakar for secretarial assistance and Mr Gokarna and Mr Omprakash Chaudhary for their technical assistance.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Epidemiology of cancer pain and factors influencing poor pain control. Am J Hosp Pallait Care. 2004;2:137-42.
- [Google Scholar]
- Pharmacological management of part 1: Better-studied neuropathic pain disease. Pain Med Suppl. 2004;5:28-47.
- [Google Scholar]
- Management strategies for the treatment of neuropathic pain in the elderly. Drugs Aging. 2002;19:929-45.
- [Google Scholar]
- Antiepileptics and treatment of neuropathic pain: Evidence from animal models. Curr Pharm Des. 2005;11:2961-76.
- [Google Scholar]
- Neuromodulating drugs for the symptomatic treatment of neuropathic pain. Curr Pain Headache Rep. 2004;8:212-6.
- [Google Scholar]
- Oxacarbazepine, topiramate, zonisamide and levetiracetam: Potential use in neuropathic pain. Am J Geriatr Pharmacother. 2003;1:18-37.
- [Google Scholar]
- Current pharmacological approach to treating neuropathic pain. Curr Pain Headache Rep. 2004;8:15-8.
- [Google Scholar]
- Antiepileptic drugs: Indication other than epilepsy. Epileptic Disord. 2004;6:57-75.
- [Google Scholar]
- Advances in the management of neuropathic pain. Phys Med Rehabil Clin N Am. 2001;12:447-59.
- [Google Scholar]
- Comparison of antiepileptic drugs tiagabine, lamotrigine and gabapentin in mouse models of acute, prolonged and chronic nociception. J Pharmacol Exp Ther. 2002;302:1168-75.
- [Google Scholar]
- GABAA receptors and excitatory amino acids in the mouse spinal cord. J Pharmacol Exp Ther. 1989;248:1026-33.
- [Google Scholar]
- Clinical activity of venlafaxine and topiramate against oxaliplatin-induced disabling permanent neuropathy. Anticancer drugs. 2005;16:587-91.
- [Google Scholar]
- Effects of anticonvulsant drug gabapentin on the enzymes in metabolic pathways of glutamate and GABA. Epilepsy Res. 1995;22:1-11.
- [Google Scholar]
- Transport of gabapentin, a γ-amino acid drug, by system 1 α-amino acid transporters; a comparative study in astrocytes, synaptosomes and CHO cells. J Neurochem. 1995;64:2125-31.
- [Google Scholar]
- Antiseizure drugs. In: Katzung BG, ed. Basic and clinical pharmacology. USA: The McGraw-Hill Companies; 2004. p. :379-99.
- [Google Scholar]
- Gabapentin relieves abnormal pain sensations via a spinal site of action in a rat model of painful peripheral neuropathy. Analgesia. 1996;2:267-73.
- [Google Scholar]
- The effects of novel antiepileptic drugs in rat experimental models of acute and chronic pain. Eur J Pharmacol. 1997;324:153-60.
- [Google Scholar]
- Effects of systemic carbamazepine and gabapentin on spinal neuronal responses in spinal nerve ligated rats. Pain. 1998;75:261-72.
- [Google Scholar]
- Gabapentin suppresses ectopic nerve discharges and reverses allodynia in neuropathic rats. J Pharmacol Exp Ther. 1999;288:1026-30.
- [Google Scholar]
- Pharmacological studies on lamotrigine a novel potential antiepileptic drug: II neurological studies on the mechanism of action. Epilepsia. 1986;27:490-7.
- [Google Scholar]
- Effects of lamotrigine on the electrically evoked release of endogenous amino acids from slices of dorsal horn of the rat spinal cord. Neuropharmacology. 1995;34:1273-8.
- [Google Scholar]
- An in vitro investigation of the action of lamotrigine on neuronal voltage-activated sodium channels. Epilepsy Res. 1992;13:107-12.
- [Google Scholar]
- Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109.
- [Google Scholar]
- Enhanced withdrawal responses to heat and mechanical stimuli following intraplantar injection of capsaicin in rats. Pain. 1996;67:179-88.
- [Google Scholar]
- CHF3381, a novel antinociceptive agent, attenuates capsaicin-induced pain in rats. Eur J Pharmacol. 2005;519:231-6.
- [Google Scholar]
- Radiant heat and hot plate method. In: Drug discovery and evaluation. Germany: Springer- Verlag Berlin Heidelberg; 1997. p. :368-70.
- [Google Scholar]
- Spinal gabapentin is antinociceptive in the rat formalin test. Neurosci Lett. 1997;222:65-7.
- [Google Scholar]
- The effects of GABAB agonists and gabapentin on mechanical hyperalgesia in models of neuropathic and inflammatory pain in the rat. Pain. 2001;90:217-26.
- [Google Scholar]
- Gabapentin suppresses cutaneous hyperalgesia following heat-capsaicin sensitization. Anesthesiology. 2002;97:102-7.
- [Google Scholar]
- Peripheral-nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;335:75-8.
- [Google Scholar]
- Gabapentin (neurontin) and S-(+)-3-isobutylgaba represents a novel class of selective antihyperalgesic agents. Br J Pharmacol. 1997;121:1513-22.
- [Google Scholar]
- Antinociceptive activity of N-methyl-D-aspartate receptor agonist N-(2-Indanyl)-glycinamide hydrochloride (CHF3381) in experimental models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;306:804-14.
- [Google Scholar]
- Advances in neuropathic pain: Diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524-34.
- [Google Scholar]
- The evolving role of antiepileptic drugs in treating neuropathic pain. Neurology. 2002;55(5 suppl 1):S41-6.
- [Google Scholar]
- Antidepressants and antiepileptic drugs for chronic non-cancer pain. Am Fam Physician. 2005;71:483-90.
- [Google Scholar]






