Translate this page into:
Tuberculous lumbar arachnoiditis mimicking conus cauda tumor: A case report and review of literature
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Tuberculous spinal arachnoiditis involving cauda equina is rare. A patient with lumbar tuberculous arachnoiditis in the absence of both vertebral and meningeal tuberculosis, which was mimicking spinal intradural extramedullary tumor is described here. Diagnosis was made based on intraoperative findings and was confirmed by histopathology. Surgical decompression along with a combination of steroid and antitubercular therapy resulted in a good outcome. At 3 months follow-up, the patient regained bladder control and was able to walk with support. Clinical features, magnetic resonance imaging, and intraoperative findings are described. Pathology and the relevant literature are discussed. Based on the patient's clinical and radiologic findings, it was believed that the patient had a conus cauda tumor and was operated on. Histologic examination of the mass revealed tuberculoma. Surgical decompression followed by antituberculosis medication resulted in good outcome. Hence tuberculous arachnoiditis should be considered in differential diagnosis of conus cauda tumors.
Keywords
Conus cauda tumor
tuberculous spinal arachnoiditis
radiculomyelitis
Introduction
Tubercular arachnoiditis and radiculomyelitis are the important causes of infectious spinal arachnoiditis.[1] The thoracic spinal cord is the most frequently involved, followed by the lumbar and cervical spinal cord.[2] Several atypical forms of tuberculous spinal arachnoiditis giving rise to confusing diagnosis are well known. We are reporting a case of conus cauda arachnoiditis mimicking as a tumor without any history of systemic tuberculosis or vertebral body lesions. We aim to illustrate the difficulties in the diagnosis and management of this potentially curable disease by reviewing the literature.
Case Report
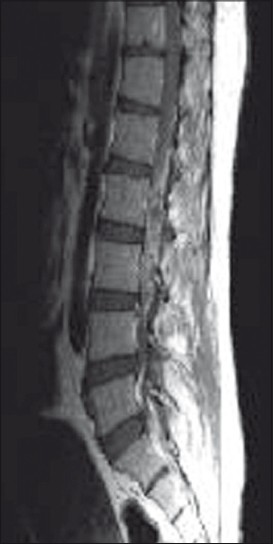
A 40-year-old man presented with dull aching, progressively increasing back pain, which was radiating to the left lower limb along the posterior–lateral aspect of the buttock up to the foot for 3 months. This was accompanied with tingling and parasthesia of both the lower limbs. One month following illness, he developed asymmetric onset of weakness in both the lower limbs involving proximal and distal muscles. He became bedbound within 15 days. He noticed numbness in the lower half of the body, including the perianal areas. He also developed bladder disturbances with constipation and erectile dysfunction for 1 week before admission. On clinical examination, he had asymmetric lower motor neuron type paraparesis with bladder and bowel involvement. Sensory loss (30%–40%) was present below L1 dermatome, including perianal region. Spinal tenderness and deformity were absent. He had normal hemogram and ESR. X-rays of the lumbosacral spine and chest were normal. Contrast MRI (lumbosacral) revealed an ill defined lesion (7.5 cm long and 1.5 cm thick) in the intradural space at L1–L4, which was isointense in T1W and heterointense in T2W [Figure 1]. The lesion demonstrates near homogenous enhancement [Figures 2 and 3]. The conus was bulky with abnormal signals. He underwent L2–L3 laminectomy and subtotal decompression of mass. Peroperatively, there was yellowish white lesion extending from L2 to L4, completely filling the spinal canal. This was adherent to the dura all around and the nerve roots. Histopathological examination of the resected mass revealed a tuberculoma with central zone of caseous necrosis [Figure 4a] walled in by granulation tissue composed of epitheloid cells, lymphocytes and Langhan's giant cells [Figure 4b]. The overlying dura was adherent, and infiltrated by histiocytes and multinucleate Langhan's giant cells. [Figure 4]. The patient was started on antitubercular medication and after 3 months he showed excellent improvement. He was able to walk without support and regained bladder control partially.

- MRI LS spine T2W sagittal cut showing heterointense lesion in the conus cauda
- MRI LS spine T1W sagittal (contrast) showing L1–L4 homogenous enhancing lesion
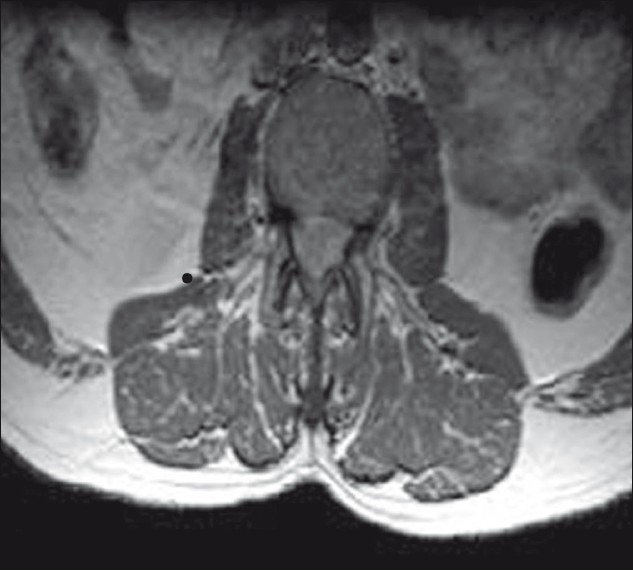
- MRI LS spine T1W axial (contrast) showing widening of cord with homogenously enhancing lesion, no subarachnoid space seen around the cord.
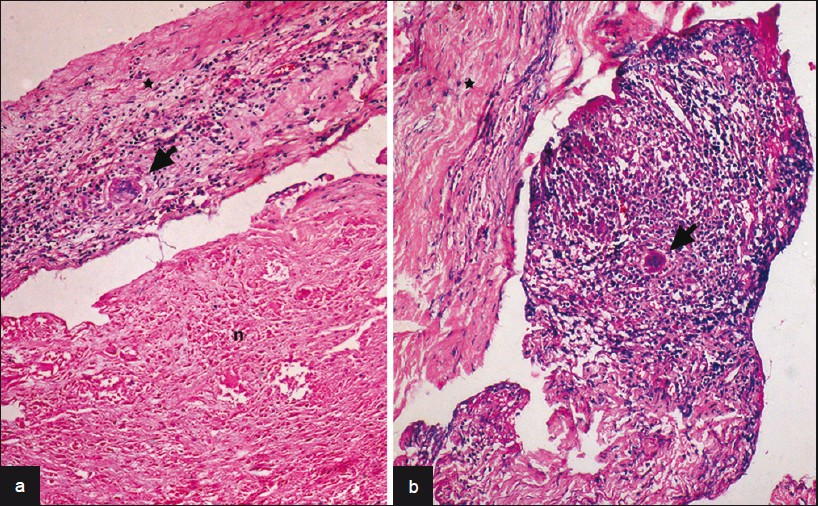
- (a) Large zones of caseous necrosis (n) seen with overlying dura infi ltrated by epitheloid cells and Langhan's giant cells (arrow) [a: HEx240, b:HEx120], (b) Microphotograph shows tuberculous granulation tissue with Langhan's giant cells (arrow) adherent to overlying infl amed dura (asterix)
Discussion
TB infection of spine occurs in the form of tuberculous spondylitis, intradural tuberculosis, and tubercular myelitis in the decreasing frequency. Intradural tuberculosis has been variously termed as intradural extramedullary tuberculosis, spinal arachnoiditis, and chronic adhesive arachnoiditis. It has been suggested that all these atypical forms of tuberculosis should be designated as tuberculous radiculomyelopathy (TBRM).[3]
TBRM may develop from 3 different sources.
-
Primary TB lesion arising in the spinal meninges.
-
A downward extension from the intracranial TB meningitis.
-
A secondary spread from adjacent vertebrae disease.
Among these, downward spread from TBM is most common. The thoracic cord is more frequently involved, followed by lumbar and cervical cords.
TBRM passes through 3 stages.[4]
-
Radiculitis—inflammation of pia arachnoid with associated hyperemia and swelling of roots.
-
Arachnoiditis—progressive fibroblast proliferation and collagen deposition leading to nerve root adhesions to each other and pia arachnoid.
-
Adhesive arachnoiditis—dense collagen deposition with encapsulation of atrophied nerve roots.
The case described here belongs to the second stage. Our case is having several atypical features as there was extensive lumbar involvement from L1 to L4 and there was no primary focus either in the brain or in adjacent vertebral bodies.
The clinical signs and symptoms are mostly limited to monoradicular or polyradicular pain syndromes that may be accompanied by motor and sensory deficits. Symptoms usually evolve over several years, although rapidly evolving cases have been described.[5] This rapid onset of symptoms, as happened in our case (<3 months) led us to the misdiagnosis of intradural tumor rather than arachnoiditis.
Classically, 3 MR patterns of arachnoiditis have been described involving cauda equina.[6]
Type 1: Central type—roots are clumped to the centre of thecal sac.
Type 2: Peripheral type—roots are adherent to the margins of the dural sac.
Type 3: Adherent roots to one side of thecal sac resembling a soft tissue mass.
MRI of our case is of type 3 showing adherent roots filling entire subarachnoid space resembling the conus cauda tumor. Differential diagnoses of contrast enhancing tumors in the conus cauda lesions in this age group include myxopapillary ependymoma, schwannoma, and paraganglioma.
Preoperatively, dura was thick, roots of cauda equina were adherent to each other and to the overlying dura. The roots of cauda equina were entangled in the granulation tissue. Similar intraoperative findings have been reported by Tanriverdi et al and Sreeharsha et al,[7] for spinal arachnoiditis.
Treatment of TBRM is mainly medical (9–12 months). Surgery should be considered only when HPE confirmation is required or there is evidence of spinal cord compression with neurologic deficit or spinal instability. Treatment of spinal tuberculous arachnoiditis may be medical or surgical. Medical treatment remains the mainstay of the treatment. Antituberculosis therapy with a combination of drugs should be started once the diagnosis is established.[8] Previous studies have shown a promising outcome with intrathecal hyaluronidase—an enzyme that hydrolyses the glucosaminidic bonds of hyaluronic acid and other mucopolysaccharides of the ground substance.[9] High-dose corticosteroid is another efficient adjuvant medical treatment, either given orally or, rarely, via the intrathecal route.[1011] The entire course of therapy should continue for at least 9–12 months.[12]
The outcome of treatment has been unpredictable, with some reports observing good recovery[213] and some reporting unfavorable outcomes after surgical decompression.[714] However, a few case studies showed good recovery after surgical decompression. So surgery—decompressive laminectomy—should be considered if histologic diagnosis is necessary or there is evidence of spinal cord compression with neurologic deficit or spinal instability.[15]
Conclusions
A possibility of tuberculous arachnoiditis should be suspected despite the absence of primary focus anywhere in the body, particularly in TB endemic regions.
We emphasize the need for careful interpretation of MRI images and that spinal arachnoiditis may closely resemble a spinal cord tumor.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Spinal infection and inflammatory disorders. In: Atlas SW, ed. MRI of the Brain and Spine (3rd ed). Philadelphia: Lippincott Williams and Wilkins Publishers; 2002. p. :1855-969.
- [Google Scholar]
- Tuberculous meningitis with spinal tuberculous arachnoiditis. Hong Kong Med J. 2003;9:59-61.
- [Google Scholar]
- Spinal meningitides with radiculo-myelopathy: Clinical and radiological features. J Neurol Sci. 1969;8:239-60.
- [Google Scholar]
- Intradural spinal tuberculosis in the absence of vertebral or meningeal tuberculosis: A case report. J Orthop Surg. 2006;14:71-5.
- [Google Scholar]
- Hyaluronidase as an adjuvant in the management of tuberculous spinal arachnoiditis. J Neurol Sci. 1991;102:105-11.
- [Google Scholar]
- Diagnosis and management of tuberculous paraplegia with special reference to tuberculous radiculomyelitis. JNeurol Neurosurg Psychiatry. 1979;42:12-8.
- [Google Scholar]
- Tuberculous radiculomyelitis complicating tuberculousmeningitis. Clin Infect Dis. 2000;30:915-21.
- [Google Scholar]
- Tuberculosis. In: Scheld MW, Whitley RJ, Durack DT, eds. Infections of the central nervous system (2nd Ed). Philadelphia: Lippincott-Raven Publishers; 1997. p. :428-36.
- [Google Scholar]
- Intradural extramedullary tuberculoma of the spinal cord. J Korean Med Sci. 2000;15:368-70.
- [Google Scholar]
- Communicating syringomyelia following cured tuberculous meningitis. J Neurol Sci. 1974;23:185-97.
- [Google Scholar]
- Diagnosis and neurosurgical treatment of tuberculous disease of the CNS. Neurosurg Rev. 1983;6:111-7.
- [Google Scholar]






