Translate this page into:
Magnetic resonance “flip-flop” in idiopathic intracranial hypertension
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Idiopathic intracranial hypertension (IIH) is a headache syndrome with raised CSF pressure in the absence of an intracranial mass lesion. Though earlier confined to excluding intracranial lesions, magnetic resonance imaging (MRI) in recent years has been shown to identify intracranial changes from prolonged raised CSF pressure, suggestive of IIH. We present the MRI and TOF (time-of-flight) venography findings involving the orbit, sella tursica and cerebral venous structures in a 45-year-old lady with IIH and illustrate their reversibility (“flip-flop”) following CSF drainage. Our case highlights the role of imaging in evaluation and follow-up of patients with IIH, without the need for repeated lumbar punctures to monitor pressures.
Keywords
Idiopathic intracranial hypertension
intracranial pressure
magnetic resonance imaging
TOF venography
Introduction
Idiopathic intracranial hypertension (IIH), previously termed pseudotumor cerebri and benign intracranial hypertension, is a syndrome of increased intracranial pressure (ICP) of unknown etiology, without clinical, laboratory or radiological evidence of intracranial pathology.[1] While the exact pathogenesis remains unclear, the chronic increased ICP in this condition may lead to morphological intracranial changes. Though traditionally performed to exclude lesions that produce intracranial hypertension, imaging in recent years has been shown to detect changes involving the orbit, sella and sinovenous system, providing important clues to the diagnosis. We describe the case of a lady with chronic headache, where magnetic resonance imaging (MRI) showed features suggestive of IIH, and in addition, highlight the dramatic reversal of these findings following CSF drainage, allowing diagnosis to be made with certainty.
Case Report
A 45-year-old housewife presented to the neurology outpatient department with chronic headache for 10 years and recent aggravation for 1 month. Positive history of nausea and vomiting and intermittent diplopia were also associated. Though she ignored these symptoms for long, they now hampered her daily household activities. Neurological examination was normal. Ophthalmological examination showed 6/6 vision in both the eyes, a normal fundus and no visual field defect on perimetry. MRI of the brain ruled out an intracranial mass. Saggital oblique images through the optic nerve revealed distended perioptic subarachnoid space as well as buckling of the orbital portion of the optic nerve [Figure 1a]. A partial empty sella with postero-inferiorly compressed pituitary having a concave superior border and posteriorly displaced infundibulum was also noted [Figure 2a]. TOF venography showed paucity of the cortical veins and non-visualization of the right transverse sinus (TS) [Figure 3a]. A provisional diagnosis of IIH was made. Lumbar puncture (LP) done following the MR study showed raised opening pressure of CSF of 28 cm of water. Twenty milliliters of CSF fluid was removed and sent for biochemical and microbiological examination, which was normal. Following CSF withdrawal, significant improvement in symptoms was noted. Repeat MRI done 3 days later showed straightening of the intraorbital optic nerve and reduction in perioptic subarachnoid space [Figure 1b]. In addition, restoration of the infundibular position with a normal appearing pituitary having a flat superior margin was seen [Figure 2b]. MR TOF showed improved visualization of the cortical veins and right TS [Figure 3b]. The patient was discharged on acetazolamide, and on follow-up at 2 and 6 months, was asymptomatic.

- T2 fat saturated oblique saggital image through the optic nerve (a, b) shows the optic nerve buckling with prominent perioptic subarachnoid space (a). Post-LP, the optic nerve straightens with some reduction in the perioptic fluid (b)
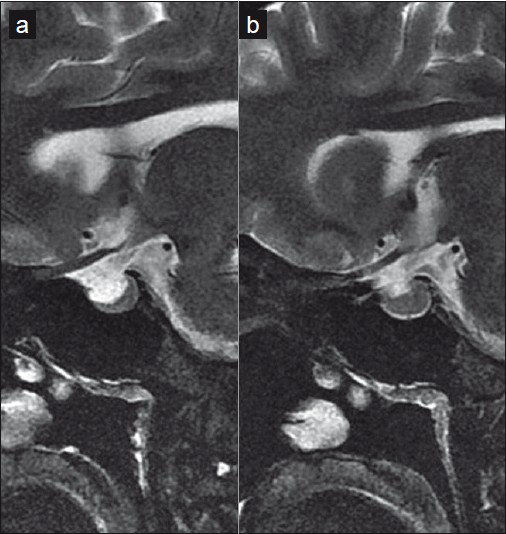
- Saggital images of the sella show "partial empty sella" (a), which, following CSF drainage, is normalized (b)
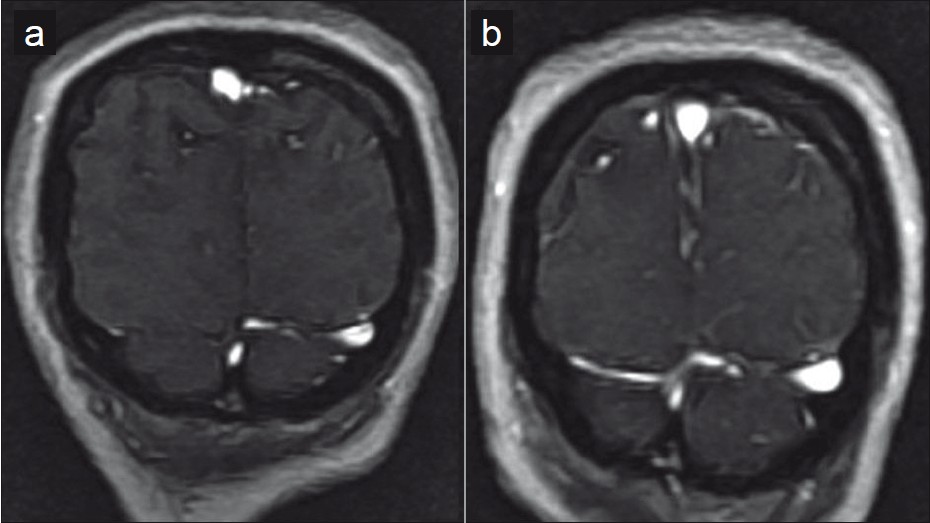
- Paucity of the cortical veins and non visualized right transverse sinus (a) is reversed following CSF drainage with distension of the sinuses and better visualization of the cortical veins (b) in this TOF venogram.
Discussion
The pathogenesis of the raised ICP in IIH is poorly understood. Possible explanations suggest a lesion of the hematoencephalic barrier with increased interstitial fluid. Increased movement of the interstitial fluid into the ventricles with equivalent resorption of CSF and subsequent rapid venous efflux maintains the flow of intracranial fluids, allowing for the raised ICP. This increase in ICP is chronic and extremely slow.[2]
Changes observed in the orbit in IIH are a consequence of the direct transmission of the raised CSF pressure. These may be appreciated on MR imaging as prominent perioptic subarachnoid space and vertical orbital optic nerve tortuousity. Both these findings were demonstrated in our patient. Additional orbital findings include intraocular protrusion of the prelaminar optic nerve and posterior scleral flattening.[3] With severe or chronic pressure, permanent degeneration of varying degrees may be seen in the optic papilla.[4] Similarly, owing to the progressive rise of pressures in patients with IIH, sellar changes may be seen. “Partial empty sella” results from arachnocele herniation through a defect in the diaphragma sella with subsequent pituitary compression and posterior infundibular stalk displacement.[5]
Acetazolamide and other diuretics including furesemide have been used in the medical management of IIH. Lumboperitoneal shunting may be required in cases unresponsive to medication, while patients with deteriorating vision may need optic nerve sheath fenestration.[16] Both the orbital and pituitary changes have shown reversibility following CSF drainage.[57] This dramatic “flip-flop” with straightening of the optic nerve, normalization of the pituitary appearance and restoration of the pituitary stalk position was similarly observed in our patient following the LP.cc
MR imaging in IIH may additionally show bilateral TS narrowing which may cause venous outflow obstruction. Whether the TS narrowing is the cause or effect is still uncertain. Theories have implicated congenital stenosis as the occasional primary cause of IIH. As an effect of IIH, stenosis may present either as a long, smooth, tapered narrowing attributed to cerebral baedema, or as an fd raised CSF pressures.[8] Venography in our patient showed paucity of the cortical veins in the pre-LP MR with improved visualization post-LP. Stenosis of the right TS was also relieved following the LP. Rohr et al, proposed MRV pre- and post - CSF diversion in suspected IIH patients to distinguish reversible and fixed transverse sinuses stenosis.[9]
In conclusion, MR imaging has an important role in evaluation and follow-up of patients with IIH, avoiding repeated lumbar punctures to monitor pressures. Signs in IIH involving the optic nerve, posterior sclera, pituitary, and sinovenous system are a direct consequence of the longstanding raised ICPs in this disease. The reversibility of these signs on MR and TOF venogram following CSF drainage not only confirms the diagnosis of IIH but also suggests a decrease in CSF pressure indicating a positive response to therapy. In addition, it also aids in differentiating venous sinus thrombosis from reversible stenosis in IIH.
Source of Support: Nil
Conflict of Interest: None declared.
References
- What is new about idiopathic intracranial hypertension? An updated review of mechanism and treatment. Cephalalgia. 2006;26:384-99.
- [Google Scholar]
- Idiopathic intracranial hypertension review and hypothesis of the pathogenesis. Res J Med Med Sci. 2006;1:104-8.
- [Google Scholar]
- Magnetic resonance imaging in pseudotumor cerebri. Ophthalmology. 1998;105:1686-93.
- [Google Scholar]
- MR of optic papilla protrusion in patients with high intracranial pressure. AJNR Am J Neuroradiol. 1996;17:665-8.
- [Google Scholar]
- Reversible empty sella in idiopathic intracranial hypertension: An indicator of successful therapy? AJNR Am J Neuroradiol. 1996;17:1953-6.
- [Google Scholar]
- Diagnosis and management of benign intracranial hypertension. Arch Dis Child. 1998;78:89-94.
- [Google Scholar]
- MR imaging of idiopathic intracranial hypertension. AJNR Am J Neuroradiol. 2001;22:196-9.
- [Google Scholar]
- Idiopathic intracranial hypertension: The prevalence and morphology of sinovenous stenosis. Neurology. 2003;60:1418-24.
- [Google Scholar]
- Reversibility of venous sinus obstruction in idiopathic intracranial hypertension. AJNR Am J Neuroradiol. 2007;28:656-9.
- [Google Scholar]






