Translate this page into:
Objective assessment of utility of intraoperative ultrasound in resection of central nervous system tumors: A cost-effective tool for intraoperative navigation in neurosurgery
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Localization and delineation of extent of lesions is critical for safe maximal resection of brain and spinal cord tumors. Frame-based and frameless stereotaxy and intraoperative MRI are costly and not freely available especially in economically constrained nations. Intraoperative ultrasound has been around for a while but has been relegated to the background. Lack of objective evidence for its usefulness and the perceived “user unfriendliness” of US are probably responsible for this. We recount our experience with this “forgotten” tool and propose an objective assessment score of its utility in an attempt to revive this practice.
Materials and Methods:
Seventy seven intraoperative ultrasound (IOUS) studies were carried out in patients with brain and spinal cord tumors. Seven parameters were identified to measure the “utility” of the IOUS and a “utility score” was devised (minimum 0 and maximum 7). Individual parameter and overall scores were calculated for each case.
Results:
IOUS was found to be useful in many ways. The median overall score was 6 (mean score 5.65). There were no scores less than 4 with the majority demonstrating usefulness in 5 or more parameters (91%). The use of the IOUS significantly influenced the performance of the surgery in these cases without significantly prolonging surgery.
Conclusions:
The IOUS is a very useful tool in intraoperative localization and delineation of lesions and planning various stages of tumor resection. It is easy, convenient, reliable, widely available, and above all a cost-effective tool. It should be increasingly used by neurosurgeons in the developing world where costlier intraoperative localization and imaging is not available freely.
Keywords
Cost-effective
intraoperative imaging
intraoperative ultrasound
scoring system
utility
Introduction
Successful extirpation of tumors of the brain and spinal cord has always remained a challenge. This is especially true for intra-axial subcortical tumors situated in the vicinity of eloquent areas. Elaborate microsurgical techniques have been described for facilitating resection of intra-axial tumors.[12] However, these techniques are possible to utilize only when the location of the underlying mass can be ascertained. Historically, the neurosurgeon has had to rely on anatomical approximations in calculating the tumor location and extent with respect to vital areas. Intraoperatively crude and ambiguous visual and tactile cues (widening of gyri, discoloration, and consistency of the tissues) and often “blind” exploratory procedures (probing/tapping using cannulas) are utilized to localize subcortical lesions. This introduces the risk of error with the potential for neurological insult. The past decade has seen tremendous advances in technological adjuncts which aid the neurosurgeon in ensuring safe, yet adequate surgery for such patients. These include adjuncts for tumor localization and margin delineation such as frame-based and frameless (neuronavigation/image-guided surgery) stereotactic devices. However, besides the cost involved (which is a severe limiting factor in developing nations), both techniques lack real-time information and rely on previously obtained CT/MR based image information which may have changed at the time the surgical procedure is performed. This disadvantage is overcome by using intraoperative imaging to guide the surgical procedure. Intraoperative MR imaging has been touted as a revolutionary tool to fulfill this need. However, this technology is prohibitively expensive thereby preventing its widespread application in neurosurgical setups the world over. Real time ultrasound was used for intraoperative localization in neurosurgery way back in the late 1970s, much before the MRI was even available for diagnostic purposes.[3] In the intraoperative setting the US has potential use and wider application especially in neuro-oncology. The range of applications and the possible roles the IOUS can play in determine its “utility.”
We recount our experience with IOUS in surgical resection of brain and spinal cord tumors. This analysis was aimed at assessing the utility of the IOUS in an objective manner. We attempted to assess the scenarios in which the IOUS could be useful and if so, in what ways would this be helpful. No attempt was made in this present analysis to assess the accuracy and efficacy of the IOUS.
Materials and Methods
A retrospective analysis of prospectively collected data was performed. The study was approved by the institutional review board. Two hundred ninety nine cases of surgical resection of brain and spinal tumors were performed between January 2007 and December 2009. We started using IOUS only much later in our practice. IOUS was used in 77 procedures (75 subjects) for various CNS tumors. The ultrasound equipment used was a basic portable unit Capasee II (Toshiba Corp., Tochigi Ken, Japan) with a linear 7 MHz frequency transducer with a small foot plate (PVF-738 F, Toshiba Corp, Japan) initially and Sonosite M Turbo machine (Sonosite Inc, Bothell, WA, USA) with a variable frequency (13–6 MHz) 25-mm footprint broadband linear array transducer (L25x), later on. Surgery was planned as is routinely done in our department reviewing the imaging to decide the route of approach. IOUS was utilized whenever the need for intraoperative imaging was deemed desirable by the operating surgeon (AVM). The radiologist accompanied the neurosurgeon during the procedure. In each of these cases, the insonation was performed both before and after dural opening. The selected probe was draped in a sterile cover filled with sterile jelly and all air bubbles were eliminated. Sterile irrigation with saline was employed during the procedure to ensure optimal coupling. The probe frequency was adjusted (with the variable frequency probe) to suitably insonate both superficial and deep structures as required.
Utility scoring system
We identified various parameters which reflect different aspects of the perceived utility of the IOUS. These were assessed and documented in the surgical notes. A scoring system was devised based on these parameters. Each parameter was given a score of 0 [no utility] or 1 [useful] [Table 1]. These individual scores were totaled to obtain an overall “utility score” (maximum 7 and minimum 0). Our scoring system assessed the utility of the IOUS, i.e., to answer the question “was the ultrasound useful in guiding/modifying a particular stage of the surgical procedure?” The efficacy and efficiency of the technique were not assessed in this analysis. A prospective observational study has been initiated to objectively asses the efficacy and will be reported subsequently.
Results
Seventy seven IOUS procedures were carried out in 75 patients (one patient with cerebral metastases from a testicular germ cell tumor was operated three times). All cases were elective surgeries. Sixty seven were supratentorial cranial tumors, 2 posterior fossa lesions and 8 were spinal intradural tumors. Sixteen patients had undergone some treatment prior to the present surgical intervention. Radical resection was performed in 32 (76%) patients. Histologically, the majority were gliomas [Table 2].

In all the cases, IOUS was found useful in many ways. The “utility scores” were shown in Table 3. The IOUS was useful by most counts, except for craniotomy planning. The median score was 6 and the mean overall utility score was 5.65. There were no scores less than 4, i.e., to say that in each case the IOUS was deemed useful in at least 4 out of 7 parameters, the majority demonstrating usefulness in 5 or more parameters (91%) [Table 4]. Operative times were not significantly prolonged (the setup and procedure on an average extended surgery by 20–30 min).To illustrate the role of IOUS in different scenarios we hereby describe representative cases.

Metastases
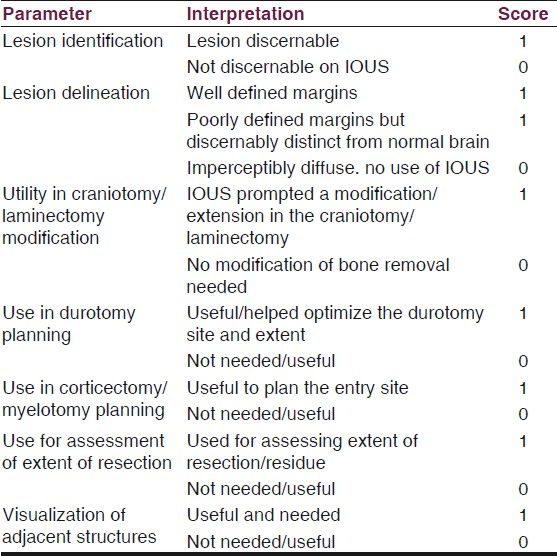
An elderly lady (previously treated for adenocarcinoma ovary) presented with focal seizures and recent onset hemiparesis. MRI brain revealed a well-defined heterogeneously enhancing lesion in the left posterior frontal region close to the motor strip [Figure 1]. At surgery, after craniotomy the lesion was not visible on the surface. Blind probing and exploration in this region close to the motor strip would have been disastrous. We used the IOUS and located the lesion exactly [Figure 1]. A restricted durotomy was made and the lesion was entered through a safe area and completely excised. The patient improved postoperatively and was referred for adjuvant therapy.
- Left frontal metastasis. Contrast MRI (left and centre panels) showing the solid-cystic mass. IOUS (right) showing the US morphology similar to the MRI
Advantage
IOUS allowed exact localization of the subcortical tumor, planning of the site of entry and complete resection, without added neurological morbidity. Moreover, the tumor morphology as seen on MRI (solid-cystic) was excellently replicated on the IOUS [Figure 1].
Low grade glioma
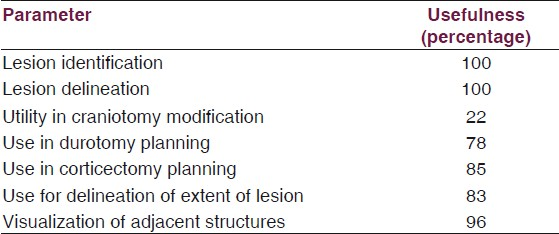
A young male presented with history of late onset focal seizures refractory to medication and no neurological deficits. MRI brain showed a well-defined nonenhancing lesion in the medial superior frontal gyrus on the left side abutting the motor strip [Figure 2]. An awake craniotomy was planned. At surgery, there was widening of the superior frontal gyrus. However, there was no morphological delineation of the tumor. IOUS demonstrated the presence of the hyperechoic mass in the medial aspect of the superior frontal gyrus [Figure 2]. The lesion was approached via the interhemispheric fissure constantly monitoring motor function and radically excised. IOUS again showed residual tumor posteroinferiorly. However, attempts at tumor manipulation here produced transient foot weakness and further resection was stopped. Postoperatively patient had mild foot weakness which improved over a few days. Postoperative MRI revealed minimal residue (as predicted by the IOUS) and adjuvant RT was planned.

- Left frontal low-grade glioma. T1 (left panel) and T2-weighted (centre panel) axial MRI showing a diffuse lesion with central cyst. IOUS picture (right panel) showing the uniformly hyperechoic lesion with cystic component
Advantage
Exact location and extent of the tumor was ascertained. Once again lesion morphology (solid with small central cyst) was well captured by the IOUS. Extent of resection could be planned and correlated well with postoperative MR.
High grade glioma
An elderly lady presented with seizures and mild hemiparesis. MR revealed what appeared to be two contiguous lesions in the posterior frontal region, one a relatively solid enhancing medially placed component with another adjacent laterally placed predominantly cystic part [Figure 3]. At surgery the solid component was easily reached via a superior frontal gyrus approach. However after it was resected the cystic part could not be identified even after repeated attempts at probing with a ventricular needle. The IOUS was then introduced and the lesion easily localized in one quadrant of the lateral resection wall. It was entered and decompressed.

- Right frontal high grade glioma. Contrast MRI sections showing two contiguous yet distinct components of the tumor
Advantage
IOUS enabled identification of cystic second component which would otherwise have been missed. This ensured adequate resection.
Recurrent/post radiotherapy lesion
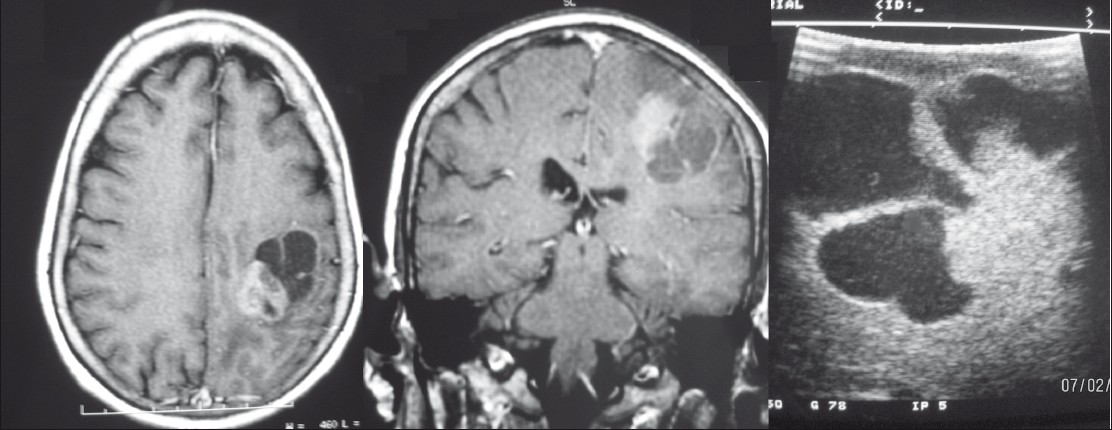
A middle-aged gentleman treated earlier for a right temporoparietal oligodendroglioma (post surgery and RT) presented 1 year later with progressive left hemiparesis and raised intracranial tension. MR revealed a large cystic lesion with enhancing solid component [Figure 4]. There was enhancement of the wall too. A combination of treatment changes and recurrence was concluded and in view of clinicoradiological progression resurgery was planned. Preoperatively the dura was adherent to the pia and the entire cortical surface was congested and appeared abnormal. IOUS was used to insonate the cystic component which was then tapped to relieve the pressure [Figure 4]. The cyst was then entered and solid area visualized from within and excised. The far wall of the cyst was insonated and no tumor tissue was detected. Hence, further resection was not performed.
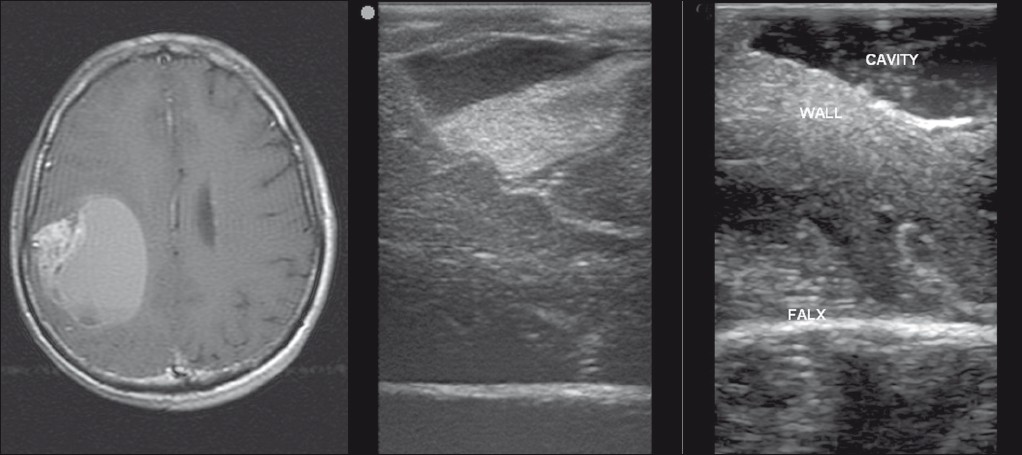
- Right parietal recurrent oligodendroglioma. MRI (left panel) showing a predominantly cystic lesion with a peripheral solid area. Preresection IOUS (centre panel) depicting the lesion and postresection (right panel) IOUS showing the resection cavity.
Advantage
IOUS permitted easy identification of the cyst and tapping it is done to gain access to the lesion. Insonation of the wall revealed no tumor and the decision was taken to leave it behind. In post-treatment cases, it is often very difficult to differentiate recurrent tumor from reactive changes. IOUS helped overcome the difficulty and planning the surgery was easier.
Spinal tumor
A 45-year-old man presented with low backache and radicular pain radiating down his left buttock. He had no neurological deficits. MRI of the spine revealed a well-defined oblong intradural extramedullary enhancing lesion at L1–L2 [Figure 5]. A limited laminectomy was performed. IOUS was used and the lesion insonated through the dura [Figure 5]. Having confirmed the adequacy of the bony exposure and the exact location of the tumor, a precise durotomy was performed and the tumor excised (histology¾paraganglioma).

- Lumbar intradural paraganglioma. MRI showing the intradural mass (left and centre panel). IOUS image (right) depicting the well delineated mass, facilitating a precise durotomy.
Advantage
IOUS provided information about the exact site of tumor and helped customize the durotomy. Extension of the laminectomy would have been considered if required.
Intraoperative doppler
A middle-aged gentleman presented with history of episodic speech arrest. His MRI revealed a heterogeneous solid-cystic mass in the left perisylvian region with areas of calcification and nodular enhancement. The left middle cerebral artery appeared close in proximity. He was planned for a resection. IOUS was used and the site of entry was ascertained through the cystic component. The lesion appeared well circumscribed. Intraoperative Doppler showed the MCA adherent to the medial surface of the mass [Figure 6]. This was not evident otherwise. The vessel was carefully dissected and the tumor was removed.

- Sylvian fissure mass. Contrast axial MRI showing a heterogeneous lesion in the sylvian fissure (left panel). IOUS image showing the underlying middle cerebral artery (arrow, center). Post-resection Doppler showing patent vessels (right panel).
Advantage
Intraoperative Doppler revealed the relationship of the vessels which could be preserved. Also, a check Doppler at the end confirmed flow and patency in the vessels.
Discussion
Ultrasound has been available as an imaging modality much before CT and MR. Though widely used in other organ system evaluations, its role as a cranial imaging modality has always remained secondary except perhaps in neonates. The primary reason is the presence of a rigid bony skull which prevents transmission of ultrasound waves to allow adequate intracranial imaging. This, however, is not a problem when the bone is removed, as is the case during surgery making intraoperative ultrasound very exciting and promising in neurosurgery. Since the initial pioneering reports of its use by Rubin and Dohrmann in the 1970s[3] and subsequent work by others,[4–13] real time B mode US showed promise and the proof of principle was established paving the way for further application in the neurosurgical operating theatre- . In fact, the use of US in neurosurgery was one of the earliest applications of US in the intraoperative setup.
Performance of IOUS does not require any major investments.[14] Routinely available scanners are good enough and suffice. The probe, however, has to be specific. It needs to have a frequency ranging from 7.5 to 10 MHz for insonating superficial structures and 3–5 MHz for deeper lesions. Such a probe can easily be added to the existing US machines (without significant financial investment) to upgrade its capability for intraoperative use. The probe footprint should preferably be small, to facilitate placement into small craniotomies and burr holes. Newer portable machines provide ease of transport coupled with superiority in resolution and variable frequency transducers makes the entire procedure more convenient and meaningful.
Since its initial use, numerous studies have demonstrated the efficacy of the US in intraoperative imaging for brain and spinal tumors.[15–23] The efficacy of the US in localizing the lesion especially for metastases and high grade tumors is good.[151718] Even with low grade diffuse gliomas, the US is better able to demarcate the hyperechoic tumor which may not be discernable on CT and difficult to localize with the naked eye at surgery.[2425] There remain concerns, however, regarding the ability of the US to resolve differences between peritumoral edema, infiltrative margin, and normal parenchyma.[26] Interestingly in a study,[27] where histological correlation was attempted, the US showed a good positive predictive value for tumor infiltrated margin. US, however, is less reliable in post-treatment cases where diffuse changes related to the treatment effect cannot be differentiated from recurrence of tumor.[28] It is also unable to provide histological characterization of lesions.[1617] US, however, is an excellent tool to differentiate solid and cystic lesions. It can also be used for real-time guidance to target lesions either for biopsy, drainage, or for catheter placements. Attempts have even been made to perform volumetric studies using the IOUS. However, its efficacy vis a vis MRI remains to be proven.[2128–30] With advances in image resolution and use of contrast US, there could be a better scope in future.[31–33]
Despite objective data regarding its efficacy the use of IOUS has not found many takers. This is partly because of the perceived “user-unfriendliness” of the IOUS. There is no doubt that neuronavigation and intraoperative MR are very good and progressive developments. However, the huge costs involved in these procedures are a major hindrance in ensuring widespread availability of the technology. Tumors affect all kinds of people (across the socioeconomic strata). An excellent and accurate technology is no good for a person for whom this expensive technology is not available or affordable. Cost defeats the purpose of the device. In addition, this scenario is very prevalent in most developing countries where even the availability of a diagnostic CT or MR is looked upon as a luxury rather than a necessity. In such a case, it becomes almost obligatory on neurosurgeons to provide alternatives. And one such cost-effective alternative available is the ultrasound.[34] Besides, in contrast to IGS, the IOUS is able to provide real-time imaging overcoming the problems of brain shift. In fact incorporation of the US in newer IGS systems[3536] testifies to the utility and superiority of the IOUS in this regard; much like a case of the poor helping the rich. Given these facts it is a pity that neurosurgeons in the developing world are either unaware of this potentially useful tool or are blinded by the glare of more glamorous technology. Few reports of its use have come from such centers.[1527] Machi et al,[15] had objectively assessed the role of IOUS in brain and spinal surgery and reported that it was useful for localization of the lesion, for delineation of tissue features as well as assessment of spatial relationships. Kumar et al,[17] employed a three-point scoring system to assess the utility of IOUS. For cranial cases, this score assessed the concurrence of the surgical plan with and without the IOUS by evaluating three parameters, viz., location, depth, and planned trajectory to the lesion. The more the discordance in the plan, the higher the score, and better the utility of the IOUS. This was primarily an assessment of the IOUS for the purpose of biopsy of deep-seated lesions. The spinal scoring system (also a three-point score) assessed different parameters (adequacy of laminectomy, adjacent neural elements, and characteristics of the tumor). The authors concluded that IOUs was useful in the cases they studied. No validation of this score has been reported though. Our scoring system is more comprehensive and applicable to both cranial and spinal cases. It incorporates more aspects to assess the utility of the IOUS at successive stages in the operation. It does not, however, assess the efficacy of the IOUS in measuring a particular parameter. Utilization of this scoring system routinely during surgeries performed with IOUS would sensitize young neurosurgeons about the potential applications of this adjunct. Increased awareness and more widespread use by neurosurgeons will no doubt enable safer neurosurgeries to be performed without having to resort to more expensive technology. Our results showed that in selected cases the IOUS was deemed to be very useful, and in more ways than one (all scores were over 3). According to our scoring system the IOUS was useful in identifying and delineating the lesions in all cases. Moreover, it was very useful in a majority of cases to plan the dural incision and choose the site of cortical entry. It also depicted surrounding anatomical details well. The only limitation was its relative ineffectiveness in helping plan the craniotomy. This is a known limitation and neuronavigation is better in planning a craniotomy. Our study reinforces the importance and utility of the IOUS especially in neuro-oncology. The use of this low cost technology should be encouraged. In short, the IOUS is a comprehensive multipurpose surgical adjunct that is useful throughout the surgery (from planning to resection) as well as in the postoperative period. There are no real disadvantages with the use of IOUS. There is virtually no risk of any mechanical brain injury or infection if used properly with the prescribed sterile precautions. The biggest advantage is that IOUS is easily available, convenient, faster (minimum delay in the surgery), and simpler to use. Moreover, as already emphasized it is very cheap compared to IGS and IOMR. No major costs of equipment and infrastructure are required. It can very well be performed using the existing systems available in all hospitals with minimum additional modification. Even the newer, sophisticated, portable IOUS devices are not as expensive.
Limitations of our study
This study, though seemingly objective incorporates a significant bias in terms of the interpretation of the various parameters (especially parameters 3, 4, and 5) used to assess the utility. Though a single neurosurgeon (AVM) recorded these observations, there could be inter-rater variability in these assessments. Whether the use of the IOUS effected a change in the surgical plan (for the better, or for the worse) is debatable. A more objective and unbiased method of assessing these parameters is preferred and has been subsequently planned as part of an ongoing prospective study. Moreover, this preliminary assessment involved a small number of patients. However, application of this scoring system in more patients would help validate the score as well as provide more robust data on the utility of this technique. Use of IOUS in consecutive patients with recording of this score would provide a better indicator of the true usefulness of IOUS in all unselected cases. Moreover, this scoring system could be similarly used to assess the utility of other intraoperative imaging/neuronavigation modalities.
The authors would like to acknowledge the support of colleagues from radiology for assistance during the performance of the clinical examinations.
Presentation: Results of this analysis were presented in part at the 7th Annual Meeting of the Asian Society of Neurooncology, Korea, 9-11 June 2010
Source of Support: Nil
Conflict of Interest: None declared.
References
- History of intraoperative ultrasound in neurosurgery. Neurosurg Clin N Am. 2001;12:155-66.
- [Google Scholar]
- Efficacy of intraoperative US for evaluating intracranial masses. Radiology. 1985;157:509-11.
- [Google Scholar]
- Intraoperative neurosurgical ultrasound in the localization and characterization of intracranial masses. Radiology. 1983;148:519-24.
- [Google Scholar]
- Use of ultrasonically guided probes and catheters in neurosurgery. Surg Neurol. 1982;18:143-8.
- [Google Scholar]
- Neurosurgical sonography: Intraoperative and postoperative imaging of the brain. AJNR Am J Neuroradiol. 1984;5:521-5.
- [Google Scholar]
- Characterization of intracranial neoplasms by CT and intraoperative sonography. AJNR Am J Neuroradiol. 1984;5:517-20.
- [Google Scholar]
- Practical application of intraoperative ultrasound imaging. Acta Neurochir (Wien). 1990;105:5-13.
- [Google Scholar]
- Intraoperative sonography in spinal surgery: Current state of the art. Neuroradiology. 1986;28:551-90.
- [Google Scholar]
- Intraoperative ultrasonography of the brain and spine. Ultrasound Quart. 2007;23:23-39.
- [Google Scholar]
- Evaluation of intraoperative ultrasound in neurosurgery. Annals Acad Med Singapore. 1993;22:422-7.
- [Google Scholar]
- Intraoperative ultrasonographic characteristics of malignant intracranial lesions. Neurol India. 2005;53:208-12.
- [Google Scholar]
- Criteria for using imaging ultrasound during brain and spinal cord surgery. J Ultrasound Med. 1984;3:155-61.
- [Google Scholar]
- Application of intraoperative ultrasound in neurological surgery. Minim Invasive Neurosurg. 2007;50:155-9.
- [Google Scholar]
- Intraoperative ultrasound imaging: Practical applicability as a real-time navigation system. Acta Neurochir Suppl. 2003;85:89-93.
- [Google Scholar]
- Ultrasound guidance in intracranial tumor resection: Correlation with postoperative magnetic resonance findings. Acta Radiol. 2005;46:743-9.
- [Google Scholar]
- Evaluation of intra-operative ultrasound imaging in brain tumor resection: A prospective study. Neurol Res. 2005;27:351-7.
- [Google Scholar]
- Anatomic visualization with ultrasound-assisted intracranial image guidance in neurosurgery: A report of 30 patients. J Am Coll Surg. 2004;199:338-43.
- [Google Scholar]
- Brain operations guided by real-time two-dimensional ultrasound: New possibilities as a result of improved image quality. Neurosurgery. 2002;51:402-11.
- [Google Scholar]
- Low grade gliomas: Comparison of intraoperative ultrasound characteristics with preoperative imaging studies. J Neuro-oncol. 1992;13:189-98.
- [Google Scholar]
- Intraoperative ultrasound imaging: comparison of pathomorphological findings in US versus CT, MRI and intraoperative findings. Acta Neurochir Suppl. 2003;85:95-9.
- [Google Scholar]
- Intraoperative ultrasound in determining the extent of resection of parenchymal brain tumours: A comparative study with computed tomography and histopathology. Acta Neurochir (Wien). 2003;145:743-8.
- [Google Scholar]
- Use of intraoperative ultrasound for localizing tumors and determining the extent of resection: A comparative study with magnetic resonance imaging. J Neurosurg. 1996;84:737-41.
- [Google Scholar]
- Correlation of intraoperative ultrasound tumor volumes and margins with preoperative computerized tomography scans: An intraoperative method to enhance tumor resection. J Neurosurg. 1989;71:691-8.
- [Google Scholar]
- A comparison between preoperative magnetic resonance and intraoperative ultrasound tumor volumes and margins. J Clin Ultrasound. 1994;22:29-36.
- [Google Scholar]
- Feasibility of contrast-enhanced sonography during resection of cerebral tumours: Initial results of a prospective study. Ultrasound Med Biol. 2007;33:571-5.
- [Google Scholar]
- Intraoperative contrast-enhanced ultrasound for brain tumors. Clin Imaging. 2008;32:419-24.
- [Google Scholar]
- Intraoperative power Doppler ultrasonography with a contrast-enhancing agent for intracranial tumors. J Neurosurg. 2005;102:295-301.
- [Google Scholar]
- Intraoperative US versus intraoperative MR imaging for guidance during intracranial neurosurgery. Radiology. 2000;215:917-8.
- [Google Scholar]
- Is the image guidance of ultrasonography beneficial for neurosurgical routine? Surg Neurol. 2007;67:579-87.
- [Google Scholar]
- Delineation of brain tumor margins using intraoperative sononavigation: Implications for tumor resection. J Clin Ultrasound. 2006;34:177-83.
- [Google Scholar]






