Translate this page into:
Moyamoya syndrome in a known case of pulmonary tuberculosis
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We report an unusual association of pulmonary tuberculosis with moyamoya syndrome in a 30-year-old Filipino female who was admitted to our hospital with a 1-week history of fever and cough. Chest X-ray showed widespread bilateral consolidation with cavity, whereas sputum was positive for acid fast bacilli (AFB). Two weeks after starting antituberculous treatment, the patient developed two episodes of loss of consciousness, which were unwitnessed. Urgent brain computed tomography (CT) showed multiple infarctions, suggesting vasculitis. The electroencephalogram showed epileptic discharges. Magnetic resonance angiography showed a picture consistent with moyamoya disease. Brain CT angiography was performed and it showed the same pictures. The patient was diagnosed with pulmonary tuberculosis-associated moyamoya syndrome. On the following days, she was discharged on antituberculous medications, antiepileptic and oral hypoglycemic treatment. After 1 year, the patient was seen in the clinic, she was well and seizure-free.
Keywords
Moyamoya disease
moyamoya syndrome
pulmonary tuberculosis
Introduction
Moyamoya disease is a rare progressive vaso-occlusive disorder of an unknown etiology. It affects the cerebral vasculature with particular involvement of the circle of Willis and the arteries that feed it. In Japanese, it means “puff of smoke” which refers to collateral circulation that develops adjacent to the stenotic vessels. The steno-occlusive areas are usually bilateral. When similar vaso-occlusive disorders occur unilaterally or are associated with underlying diseases, it is referred to as moyamoya syndrome.[12] Moyamoya alone, without the distinguishing modifier of “disease” or “syndrome”, refers solely to the distinctive findings on cerebral arteriography, independent of the cause.[2] Many disease states have been reported in association with moyamoya syndrome, which may be coincidental, but can complicate management. The coexistence of moyamoya syndrome and pulmonary tuberculosis is very rare. In this report, we have described an unusual association of pulmonary tuberculosis with a typical moyamoya syndrome in a young female patient with seizure. To the best of our knowledge, this is the first typical case of bilateral moyamoya vessels in a patient with an established diagnosis of pulmonary tuberculosis.
Case Report
A 30-year-old Filipino female admitted to our hospital with a 1-week history of fever and cough. The patient has diabetes mellitus 1 year ago, but she was not receiving any medication. Other medical histories were unremarkable. On examination, the patient looked well, not dyspneic. Her blood pressure was 110/70 mmHg, pulse rate 90 beats/min, respiratory rate 20 cycles/min and temperature 37°C. Chest examination revealed bronchial breathing with crackles, mainly on the right lung. The rest of the clinical examination otherwise was normal.
Blood investigations revealed a hemoglobin level of 13.8 g/dL, a total leukocyte count of 12,900/mm3(91.7% neutrophils, 1.7% lymphocytes) and an adequate number of platelets. Erythrocyte sedimentation rate was 53 mm/hour. Blood chemistry, liver profile and coagulation studies were within normal limits. Chest X-ray showed widespread bilateral consolidation with cavity formation, suggesting active tuberculosis infection, whereas sputum was positive for acid fast bacilli (AFB). Human immunodeficiency virus enzyme-linked immunosorbent was negative.
On the 3rd day of hospitalization, four-drug antituberculous treatment was started. To control her blood sugar, the patient was kept on sliding scale insulin.
Two weeks after starting antituberculous treatment, the patient developed two episodes of loss of consciousness, which were unwitnessed. The exact duration was unknown, but when awakened, the patient complained of severe headache and backache. Neurological examination was unremarkable.
ECG and blood chemistry (including blood sugar), complete blood count (CBC) and liver profile were normal. Lumber puncture was carried out to rule out tuberculous meningitis. Cerebrospinal fluid (CSF) was clear with an opening pressure of 40 mm H2O, and analysis of CSF revealed the following values: WBC count of 5 cells/mm3(100% lymphocytes); glucose level 3.8 mmol/L; and protein level 0.78 g/L. Gram and Zeihl-Neelsen stains were negative. Echocardiography and holter monitoring also were normal, whereas urgent brain computed tomography (CT) showed multiple infarctions without abnormal dural enhancement [Figures 1 and 2]. Screening for thrombophilia and vasculitis was carried out. Anti-thrombin III, activated protein C resistance, factor V Leiden, protein C, protein S, clotting time, prothrombin time, activated partial thromboplastin time, fibrinogen, anticardiolipin antibody, lupus anticoagulant screen, homocystine, antinuclear antibody (ANA), C3, C4, C-reactive protein (CRP) and anti-neutrophil cytoplasmic antibodies (ANCAs), all these tests were within normal limits. The electroencephalogram (EEG) showed epileptic discharges. Magnetic resonance imaging (MRI) showed infarction at the head of the left caudate nucleus, the antero-lateral aspect of putamen and intervening internal capsule [Figure 3], whereas magnetic resonance (MR) angiography showed a picture consistent with moyamoya. Brain CT angiography was performed and it showed the same pictures [Figure 4].
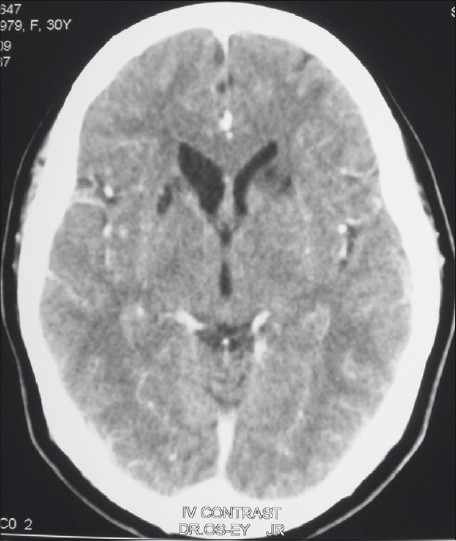
- Contrast-enhanced CT scan of the brain, showing bilateral gliotic changes in the head of the left caudate nucleus, the antero-lateral aspect of putamen and intervening internal capsule. Appearances of old infarcts are seen along the recurrent artery of Heubner
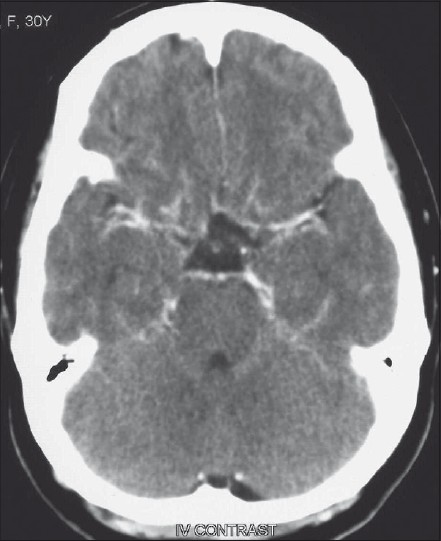
- Contrast-enhanced CT scan at the level of the basal cisterns, showing bilateral subtle enhancement along the posterior end of the gyrus rectus due to basal collaterals in association with moyamoya. There was no abnormal dural enhancement noted in the rest of the dural covering

- Coronal T2W MR showing the previous infarctions mentioned in Figure1, in addition to another old left frontal cortical infarct
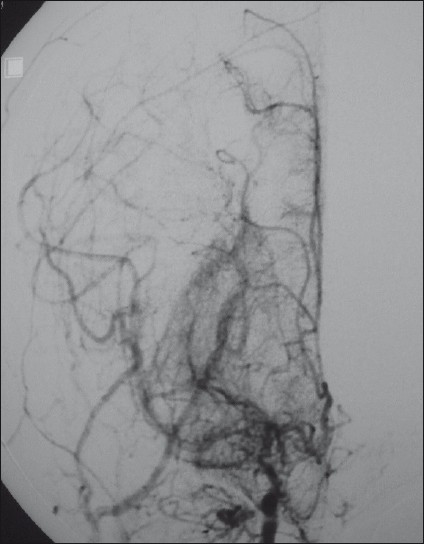
- Frontal view of a right internal carotid catheter angiogram, showing the “Puff of Smoke” appearance due to innumerable enlarged basal perforators overshadowing the attenuated anterior and middle cerebral arteries. Also, collaterals from the external carotid artery are enlarged to retrogradely fill the peripheral branches of the middle and anterior cerebral arteries
The patient was diagnosed with pulmonary tuberculosis-associated moyamoya syndrome. On the following days, she was discharged on antituberculous, antiepileptic (Leveteracetam) and oral hypoglycemic (Gliclazide) medications. After 6 weeks, CSF culture failed to grow Mycobacterium tuberculosis. One year later, the patient was seen in the clinic; she was well and seizure-free.
Discussion
Symptoms and signs of moyamoya can be attributed to changes in flow resulting from stenosis of the internal carotid artery. The patterns of presentation of moyamoya disease reported in the literature so far have shown several consistent features; it most often presents with ischemic symptoms [i.e., stroke, transient ischemic attacks (TIAs), and seizures], or symptoms due to the deleterious consequences of the compensatory mechanisms responding to the ischemia (i.e., hemorrhage from fragile collateral vessels and headache from dilated transdural collaterals).[3] Our patient presented with seizures.
The exact etiology of moyamoya disease is unknown. Some genetic predisposition is suggested because 6–10% of the patients in Japanese and US series have affected first-degree relatives. Most familial cases appear to be polygenic or inherited in an autosomal dominant fashion with incomplete penetrance.[4] On the other hand, levels of many growth factors, enzymes, and other peptides have been reported to be increased in association with moyamoya disease.[5] Therefore, it was presumed that the disease is hereditary and multifactorial.
Several diseases may be associated with moyamoya syndrome, including Grave's disease, aplastic anemia, Fanconi anemia, sickle cell anemia, lupus anticoagulant, Apert syndrome, Down's syndrome, Marfan syndrome, Turner's syndrome, von Recklinghausen disease, Hirschsprung disease, coarctation of the aorta, fibromuscular dysplasia, cranial trauma, radiation injury, parasellar tumors, hypertension, leptospirosis, AIDS and tuberculosis.[6–11]
Although a few cases of tuberculous meningitis associated with moyamoya syndrome have been reported,[1112] pulmonary tuberculosis with a typical moyamoya syndrome is extremely rare. A MEDLINE search showed that no such cases have been reported. But we found two patients with moyamoya syndrome associated with pulmonary tuberculosis, involved in a study.[13]
In this patient, the differential diagnosis of seizures may include hypoglycemia; drug (INH) induced encephalopathy and tuberculous meningitis. Normal blood sugar, disappearance of seizures despite the continuation of antituberculous medications and non-conclusive CSF study made these diagnoses unlikely. Seizures were attributed unexpectedly to moyamoya syndrome.
Based on autopsy observation of cases of tuberculous meningitis, arteritis as the cause of angiographically demonstrable narrowing and occlusion of the cerebral arteries has been stressed on by Mathew et al.[11] Moreover, cerebral arteritis associated with tuberculous foci apart from the nervous system has been reported by some authors.[1415] Based on these observations, though the pathologic examination for the cerebral vessels was not performed, we could hypothesize that cerebral arteritis is responsible for the angiographic changes in our patient.
The effective treatment of pulmonary tuberculosis-associated moyamoya syndrome has not yet been determined, as this combination of diseases is quite rare. Our patient received antiepileptic treatment and continued her antituberculous treatment. When she was seen in the clinic after 1 year, the patient was seizure-free.
In conclusion, moyamoya syndrome is a recognized cause of seizures in young adults. Patients with pulmonary tuberculosis may be particularly at risk for moyamoya, which should be considered in the differential diagnosis of patients with pulmonary tuberculosis and seizures even in the absence of active meningitis.
Source of Support: Nil,
Conflict of Interest: None declared.
References
- Clinical features, surgical treatment, and long-term outcome in adult patients with moyamoya disease: Clinical article. J Neurosurg. 2009;111:936-42.
- [Google Scholar]
- Inheritance pattern of familial moyamoya disease: Autosomal dominant mode and genomic imprinting. J Neurol Neurosurg Psychiatry. 2006;77:1025-9.
- [Google Scholar]
- New vessel formation in the central nervous system during tumor growth, vascular malformations, and Moyamoya. Curr Neurovasc Res. 2006;3:237-45.
- [Google Scholar]
- Fanconi anemia and moyamoya: Evidence for an association. Neurology. 1995;45:998-1000.
- [Google Scholar]
- Moyamoya disease in a girl with Down syndrome: Report of one case. Rev Med Chil. 2009;137:1066-70.
- [Google Scholar]
- Moyamoya syndrome associated with systemic lupus erythematosus. Lupus. 2008;17:679-82.
- [Google Scholar]
- Moyamoya disease associated with Graves disease: Special considerations regarding clinical significance and management. J Neurosurg. 2005;102:1013-7.
- [Google Scholar]
- Case of moyamoya disease in a patient with advanced acquired immunodeficiency syndrome. J Stroke Cerebrovasc Dis. 2007;16:268-72.
- [Google Scholar]
- Cerebral angiographic features in tuberculous meningitis. Neurology. 1970;20:1015-23.
- [Google Scholar]
- Risk factors of moyamoya disease in Canada and the USA. Clin Neurol Neurosurg. 1997;99:S45-8.
- [Google Scholar]
- Papulonecrotic tuberculoid secondary to Mycobacterium bovis. Arch Dermatol. 1978;114:564-6.
- [Google Scholar]
- A case of temporal arteritis due to oral cavity lesion of tuberculosis. J Res Med Sci. 2004;2:99-101.
- [Google Scholar]






