Translate this page into:
Drug interaction as cause of spontaneously resolving epidural spinal hematoma on warfarin therapy
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We present a case of a 42-year-old male, an old case of deep vein thrombosis on warfarin and other drugs like quetiapine, aspirin, diclofenac sodium, fenofibrate, atorvastatin, propanolol and citalopram for concurrent illnesses, who presented with widespread mucocutaneous bleeding and epidural spinal hematoma. The epidural bleed presented clinically as a nontraumatic, rapidly improving myeloradiculopathy. Magnetic resonance imaging (MRI) of the spine revealed an epidural hematoma at D12-L1 level. The case was managed conservatively due lack of neurosurgical facilities. The patient gained full neurological recovery on conservative management alone. This case highlights the problem of drug interaction on warfarin therapy and also an unusual spontaneous recovery of spinal hematoma. Our case was anticoagulated in the recommended therapeutic INR range of 2.2 to 2.4. Most of the similar cases reported in literature were also anticoagulated in the therapeutic range. Thus intraspinal hemorrhage is a rare but dangerous complication of anticoagulant therapy. It must be suspected in any patient on anticoagulant agents who complains of local or referred spinal pain associated with neurological deficits. Drug interactions with warfarin are common. High suspicion and immediate intervention are essential to prevent complications from intraspinal hemorrhage.
Keywords
Anticoagulant
spinal epidural hematoma
warfarin
Introduction
Spinal hematoma has been described as a clinical entity since 1850 by Tellegen and in autopsy studies as early as 1682.[1] Without adequate treatment it often leads to death or permanent neurological deficit. There is paucity of data to estimate the incidence of spinal hematoma, perhaps due to the rarity of this disorder. The incidence of spontaneous spinal epidural hematoma is about one in one million individuals per year with a male preponderance of 3:1, occurring most commonly between the ages of 42 to 52 years.[2]
No etiological factor can be identified in about one-third of cases of spinal hematomas. Spinal hematomas, occurring on anticoagulant therapy, are even more uncommon.[1] Most spinal hematomas are located dorsally to the spinal cord in the cervicothoracic and thoracolumbar regions.[3] The patients on anticoagulants are usually on drugs for other concurrent or primary illness. Importance of drug interaction with warfarin is emphasized. Early surgical decompression of the spinal cord has been recommended to reduce the extent of permanent neurological deficit.[4]
We present a follow-up case of deep vein thrombosis on warfarin therapy and other multidrug prescriptions for other concurrent illnesses, who presented with widespread mucocutaneous bleeding and epidural spinal hematoma. The unique feature of this case was the complete neurological recovery on steroids alone.
Case Report
A 42-year-old obese man presented with painful swelling of right upper limb up to the axilla of five days duration following vigorous use of the limb while lifting heavy weights. He had been on fenofibrate and atorvastatin for dyslipidemia for past two years, and on warfarin and aspirin for deep vein thrombosis (DVT) of right lower limb eight months back. He had received diclofenac sodium for pain in the arm from his primary care physician. He was a nonsmoker and teetotaller. He denied history of fever, chest pain, dyspnea, pain abdomen, gum bleeds, hematemesis, melena, or hematuria. Evaluation eight months ago for the coagulopathy was normal (Protein C, Protein S, Factor V Leiden, APTT, Anti-thrombin III, antiphospholipid antibodies, fibrinogen levels, factor VIII, IX, XI levels were all normal). Ultrasound Doppler of lower limbs during follow up six months back had revealed minimal recanalization of right saphenofemoral and popliteal veins.
Clinical examination revealed an obese individual with normal vital parameters. He had edema over dorsum of right hand with right upper limb girth three cms more than corresponding area on left. Other systems were clinically normal. Investigations revealed a normal hemogram and metabolic profile. Prothrombin time was 14 and 22 sec (control and test respectively) with INR of 2.2. Plain X-ray of the affected arm revealed soft tissue swelling but no bony abnormality. Chest X-ray and ECG were normal. A duplex scan ruled out right axillary vein thrombosis; two small hematomas, about one x one x half cm, were detected in the belly of biceps and triceps muscles. Purpuric patches appeared over the right arm. Warfarin and aspirin were discontinued.
Reversal of anticoagulation was attempted with Vitamin K and fresh frozen plasma infusions. He, however, developed pain in large joints, and large muscles of thighs and arms; previously purpuric patches became ecchymotic, fresh ecchymotic patches appeared over wrist, abdomen, arms and tongue, and developed oozing from venipuncture sites. He passed a large amount of melena. Hypovolaemic shock was corrected with parenteral fluids, whole blood and packed cells transfusions, and other supportive therapy.
History was revisited. He had been on quetiapine, propranolol and citalopram for depression / schizophrenia for last five years. This information had not been revealed by him for fear of stigma. He continued to consume these medications while in hospital. These drugs and fenofibrate were discontinued. The course was further complicated by severe low back pain and radicular pains around the lower waist. Few hours later, he developed urinary retention with loss of bladder sensations, followed few hours later by asymmetric paraparesis. Power of bilateral hip extensors was MRC grade three and right foot dorsiflexion was MRC grade four. Other muscle groups had normal power. Tone was normal. Knee jerks were brisk bilaterally and ankle jerks were normal. Right plantar response was extensor. Cremaster reflex was absent on both sides. There was 40-50% loss of pain and temperature in left leg and thigh; impaired touch and vibration in the right leg. Spinal tenderness at D12 - L1 level was present.
A diagnosis of intraspinal hemorrhage was made. There was no neurosurgical facility available at our town so surgery was not an option. Thus the patient was put on parenteral Dexamethasone. MRI thoracolumbar spine revealed an epidural hematoma at D12 - L1 vertebral level with compression of the cord and conus medullaris from right lateral aspect [Figures 1 and 2].
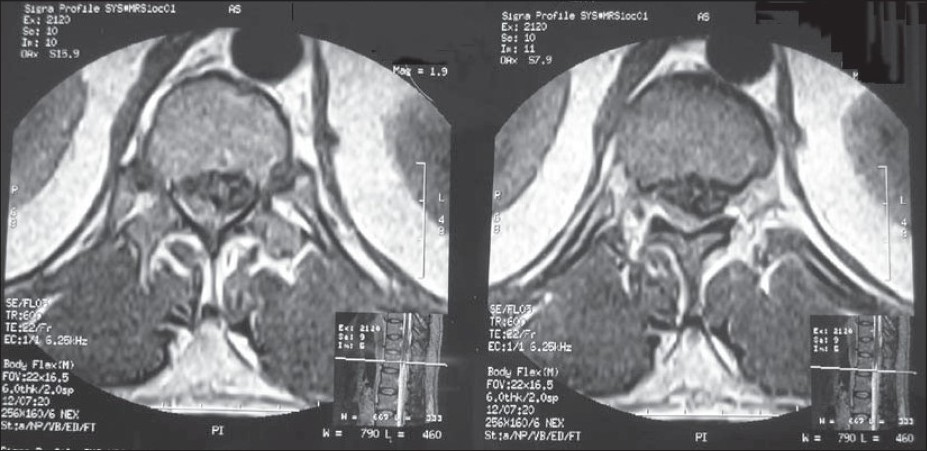
- MRI spine showing epdural hematoma at D12 - L1 vertebral level more in posterolateral aspect
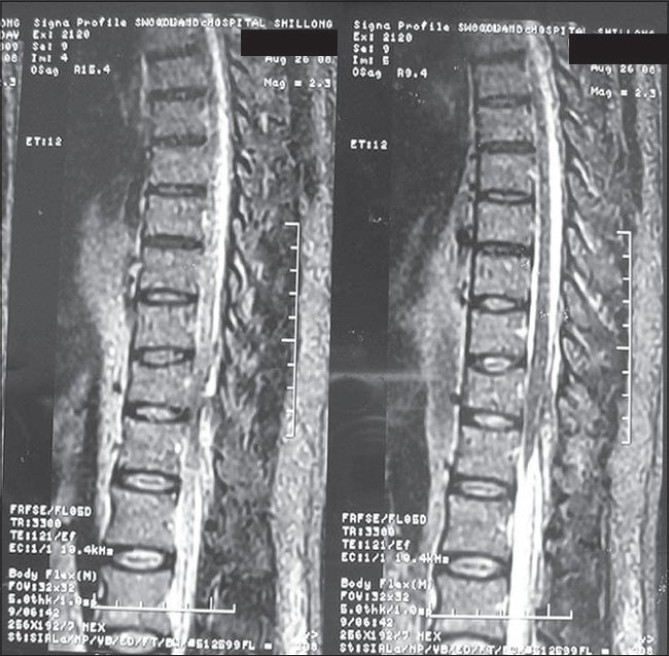
- MRI spine showing compression of the cord and conus medullaris
By day six he had complete neurological recovery, and was walking independently free of pain. Mucocutaneous bleeding had arrested. Follow-up MRI spine at eight weeks was normal.
Discussion
Spinal hematomas occurring during anticoagulant use are usually epidural or subdural in location.[5] Intramedullary hematomas and subarachnoid spinal hemorrhages are also encountered during anticoagulant therapy though much rare.[67] Dorsal and lumbar areas are most commonly involved on anticoagulant therapy.[89] The most frequent entity of acute or chronic spinal hematoma is the spinal epidural variety. Depending on the cause the epidural bleeds can be classified as idiopathic, spontaneous and secondary. As the name suggests the idiopathic variety has no cause known. The spontaneous variety accounts for 0.3 to 0.9% of the total spinal epidural bleeds.[210] The spontaneous variety is associated with risk factors like abnormalities of coagulation (due to antithrombotics, thrombolytics or conditions like hemophilia); with normal coagulation parameters but platelet dysfunction like in polycythemia; soft tissue trauma, lumbar puncture, underlying neoplasm, conditions causing raised intravascular pressure (such as sneezing, lifting weights, pregnancy, Valsalva maneuvre) and conditions with certain vascular pathologies like spinal vascular malformations, vasculitis or Paget's disease.[21112] The final common endpoint pathology is bleeding from the valveless epidural veins. The chronic variety is rare and usually located in the lumbar area.[2]
In our patient there were no predisposing factors as discussed in previous paragraph except that he was on anticoagulants. The episode happened while being hospitalized, though a straining event such as sneezing cannot be ruled out. Clinical presentation may not allow differentiation of epidural from subdural haematoma.[313] Non traumatic severe back pain or radicular pain has been classically described as the earliest and most marked symptom in spinal epidural hematoma, followed by progressive neurological deficit attributable to spinal cord and radicular compression.[29] It is noteworthy that many of the reported cases were anticoagulated in the therapeutic range.[14] Our case was also anticoagulated in the recommended therapeutic INR range of 2.2 to 2.4.
Anticoagulant therapy alone probably does not trigger spinal hemorrhage. It is likely that there is small abnormal locus together with increased pressure in the internal vertebral venous plexus in order to cause spinal hemorrhage. These two factors are thought to be the predisposing factors causing spinal hematoma in patients on anticoagulants.[1] On suspicion of intraspinal hemorrhage, anticoagulation must be reversed immediately. Emergency laminectomy and decompression of the spinal cord appear mandatory if permanent neurologic sequelae are to be minimized, though rare cases with rapid spontaneous recovery have been reported.[1415] In young patients with mild, non-progressive or rapidly improving symptoms, a trial of non-operative management may succeed.[2]
Since neurosurgical facilities were not available at this peripheral hospital, medical decompression with parenteral dexamethasone was attempted. Recovery is best in those patients who have less severe preoperative symptoms and have been decompressed early. The extensive neurological involvement and the MRI corroboration of a large epidural hematoma along with the dramatic clinical response to steroid highlight the importance of prompt clinical suspicion and early medical intervention. A low threshold to perform magnetic resonance imaging leads to a quicker diagnosis of spinal epidural hematoma. MRI, however, may be inconclusive for differential diagnosis of etiology.[11]
Drug interaction is a practical problem while using warfarin. Most antibiotics enhance the effect of warfarin by decreasing the synthesis of Vitamin K from gut commensals resulting in increased bleeding tendency. NSAIDS displace warfarin from protein binding resulting in increased effect of bleeding. Other drugs that potentiate bleeding risk with warfarin are alcohol, allopurinol, anabolic steroids, amiodarone, selective serotonin reuptake inhibitors, clofibrate, and to a lesser extent gemfibrozil, antidiabetics, antimalarials, antiplatelets, anxiolytics, disulfiram, levothyroxine, and beta blockers like propranolol. Examples of drugs which reduce the effect of warfarin are contraceptives, raloxifene, retinoids and vitamin K. Quetiapine an antipsychotic medication used in treatment of schizophrenia and bipolar disorders has been implicated in a few reports for increased bleeding effects of warfarin when administered concurrently.
In summary, intraspinal hemorrhage is a rare but dangerous complication of anticoagulant therapy. It must be suspected in any patient taking anticoagulant agent and who complains of local or referred spinal pain associated with limb weakness, sensory deficits, or urinary retention. Awareness of common drug interactions with warfarin, avoiding polypill and proxy prescriptions and the importance of taking a good drug history cannot be overemphasized. Multi drug prescriptions should be critically reviewed and tailored if incompatible with warfarin. The risk of intraspinal hemorrhage associated with anticoagulant therapy may be minimized by close monitoring and tight control of the intensity of anticoagulation.[15] A high level of suspicion, vigilance and immediate intervention can prevent major morbidity and mortality from intraspinal hemorrhage.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Spinal hematoma: A literature survey with meta-analysis of 613 patients. Neurosurg Rev. 2003;26:1-49.
- [Google Scholar]
- Spontaneous spinal epidural haematomas and the prognostic implications of interval to surgical decompression: A report of two cases. J Orthop Surg. 2009;17:216-9.
- [Google Scholar]
- Spinal subdural hematoma associated with anticoagulant therapy in a patient with spinal meningioma. Neurosurgery. 1981;8:600-3.
- [Google Scholar]
- Spinal epidural hematoma during anticoagulant therapy: A case report and review of the literature. J Neurosurg Sci. 1995;39:87-94.
- [Google Scholar]
- Thoracic intramedullary haematoma as a complication of warfarin: Case report and literature review. Aust N Z J Surg. 1991;61:789-92.
- [Google Scholar]
- Spontaneous intramedullary haematoma as a complication of anticoagulant therapy. Acta Neurochir (Wien). 1980;52:73-7.
- [Google Scholar]
- Spinal subarachnoid hemorrhage complicating oral anticoagulant therapy. Eur J Radiol. 2001;39:176-9.
- [Google Scholar]
- Spinal chronic subdural hematoma in association with anticoagulant therapy: A case report and literature review. Spine. 2006;31:184-7.
- [Google Scholar]
- Cervical hematomyelia secondary to oral anticoagulant therapy: Case report. Neuroradiology. 2001;43:1087-8.
- [Google Scholar]
- Spontaneous spinal epidural hematoma with unusual hemiparesis alternating from one side to the other side. Intern Med. 2009;48:1703-5.
- [Google Scholar]
- Spontaneous and idiopathic chronic spinal epidural hematoma: Two case reports and review of the literature. Eur Spine J. 2009;18:1555-61.
- [Google Scholar]
- Spontaneous subdural hematoma of the thoracolumbar region with massive recurrent bleed. Indian J Orthop. 2009;43:412-5.
- [Google Scholar]
- “Painless” spinal epidural hematoma during anticoagulant therapy. Neurology. 1976;26:213-25.
- [Google Scholar]
- Spinal epidural hematoma associated with oral anticoagulation therapy. Am J Phys Med Rehabil. 2004;83:220-3.
- [Google Scholar]
- Intraspinal hemorrhage complicating oral anticoagulant therapy: An unusual case of cervical hematomyelia and a review of the literature. Arch Intern Med. 2000;160:237-40.
- [Google Scholar]






